Fourteen students at the Gerstner Sloan Kettering Graduate School of Biomedical Sciences (GSK) successfully defended their dissertations this past year. They will be awarded PhD degrees during the school’s eighth commencement ceremony on May 29 as part of MSK’s 40th annual academic convocation.
The event recognizes students who have completed their thesis work in laboratories at MSK. This includes students graduating from Weill Cornell Graduate School of Medical Sciences as well as GSK. The ceremony also honors distinguished scientists and clinicians at MSK and beyond for their research success.
This year’s degree recipients from GSK represent the largest group of students to graduate in a given year since the school was established in 2004. Their graduation brings the total number of GSK alumni to 67.
“As these bright, talented graduates embark on the next stage of their careers, they are poised to make an impact on biomedical research,” says GSK Dean Michael Overholtzer. “They truly embody our mission to train the next generation of scientists to make discoveries that will further our understanding of human disease.”
GSK will award doctorates to Nicholas M. Adams, Steven K. Albanese, Scott J. Callahan, Mary E. Klein Dooley, Weiran Feng, Yun-Han Huang, Mehmet Erman Karasu, Edward R. Kastenhuber, Michael Langberg, Qing Li, Brian H. Santich, Kelsey R. Temprine, George L. Vaisey, and Raymond Wu. Below is a summary of their thesis projects.

I enrolled in GSK as a student in the Tri-Institutional MD-PhD Program, a physician-scientist training program jointly sponsored by Weill Cornell Medicine, The Rockefeller University, and Memorial Sloan Kettering. I completed my dissertation research under the mentorship of immunologist Joseph Sun. His lab studies natural killer (NK) immune cells and their role in the immune system’s response against infection and cancer.
People who lack NK cells often suffer complications from viral infections. One recent study identified that biallelic mutations in IRF8 coincided with severe, and in some cases fatal, viral susceptibility in three unrelated families.
Cytomegalovirus (CMV) is a viral infection commonly observed in susceptible families. NK cells express receptors to recognize CMV proteins, causing the NK cells to kill infected cells, secrete cytokines, and proliferate. Using a mouse model of CMV, we uncovered an unexpected role for IRF8 in promoting the proliferation of NK cells, a critical process by which NK cells multiply to combat this viral infection. The IRF8 function that we identified may partially explain the viral susceptibility observed in people with IRF8 mutations.
In another study, we demonstrated that the degree to which NK cells express the CMV receptor has an impact on the functional response. Furthermore, in collaboration with physician-scientist Katharine Hsu, we observed modulation in the expression of NKG2C, a human CMV receptor, on the NK cells of people who reactivated CMV after a hematopoietic stem cell transplant. This provides evidence of real-time adaptation in the human NK cell repertoire to CMV infection.
I am now completing my final two years at Weill Cornell Medical College to earn my MD degree.

I chose to pursue my graduate studies at GSK because it provides access to a phenomenal group of scientists and mentors. As a biophysicist, I am primarily interested in how protein structure is related to cell function and disease in order to develop effective treatments.
I performed my dissertation work under the mentorship of computational chemist John Chodera. His lab focuses on computational chemistry, statistical mechanics, molecular modeling, and automated biophysical experiments to identify new therapies and investigate the mechanisms of drug resistance in cancer. I was drawn to the opportunity to learn computational techniques that are based on the structure of proteins and are directly applicable to the drug discovery process.
My thesis centered primarily on two questions: How do genetic mutations in cancer cells impact their proteins’ structure and binding capabilities, and how can we predict a drug’s ability to selectively bind to specific proteins in a cell? To that end, I applied computational physics–based models to predict selectivity and resistance to small molecule kinase inhibitors.
Kinases are a family of proteins that are commonly mutated in cancer. There are more than 500 kinases in a human cell, and most inhibitors bind to a site on a protein that is common across all of these. The selectivity of a compound, or how many of these kinases a drug binds to, is a critical part of drug discovery. Poor selectivity can result in toxicity that can hurt patients and doom a drug development project.
Currently, more than 45 cancer drugs target kinase proteins. I developed computational models to better understand how to predict the selectivity of these inhibitors and the impact that mutations may have on an inhibitor’s ability to bind to a protein. This information could ultimately help doctors make decisions about which drug to give someone who presents with a new mutation that hasn’t been seen before. Much more research is needed, but my dissertation work represents one of the first steps toward realizing this possibility.
My experience at GSK allowed me to grow immensely as a scientist and choose a research path that I barely knew existed as an undergraduate. I am now working as a computational scientist at Schrödinger, a computational chemistry company that applies computer-aided techniques to active drug discovery projects.

I chose to pursue my degree at GSK because of its amazing faculty. Mentored by cancer biologist and oncologist Richard White and developmental biologist Lorenz Studer, I was able to conduct leading-edge research in an intimate setting and learn from two of the smartest and most caring people I know.
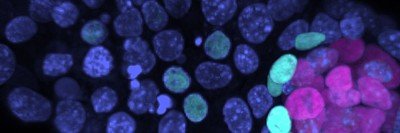
My dissertation explored how melanoma originates. Melanoma cells vary in terms of their genetics, level of pigmentation, and how quickly they grow, spread, and respond to therapy. The diversity of these cancer cells is well established but poorly understood. As a result, current treatments for melanoma do not account for these differences and yield less effective outcomes.
Recent advances have allowed scientists to characterize melanoma in greater detail and better understand its molecular signatures. Researchers have observed two distinct melanoma tumor populations, or phenotypes: One type grows slowly but is more likely to metastasize; the other tends to grow faster and exhibits more pigmentation.
One theory for these distinct phenotypes is that melanoma can initiate in different kinds of cells. We tested this hypothesis using two approaches. First, we created an animal model in which we encouraged melanoma tumors to grow in different cells within the skin of a zebrafish. To test our hypothesis in the human context, we coaxed human embryonic stem cells into skin cells and induced melanoma to grow in those cells.
We then took the tumors that developed in both the fish and human cells and compared them to melanoma that naturally occurred in patients. Interestingly, we found that melanoma from patients originated from a distinct and much narrower subtype of cells than previously assumed.
Identifying the specific phenotype of cells from which melanoma originates provides researchers with new, more accurate models for studying the disease in the laboratory setting. Our hope is to deduce ways of halting melanoma progression right at the start.
I am now working as a medical writer at BGB Group, helping to communicate science to nonscientists.

I decided to pursue my doctorate at GSK after participating in the school’s Summer Undergraduate Research Program. This experience exposed me to the incredibly diverse research being done at MSK and the supportive culture that GSK fosters to help its students thrive.
I began my training at GSK knowing that I wanted to work on research that could be translated from the bench to the bedside. I chose to complete my thesis work in the laboratory of molecular biologist Andrew Koff, where I had the opportunity to perform research in molecular biology and examine its clinical impact. His lab uses biochemical, molecular, cellular, and genetic approaches to investigate CDK inhibitors and how they accomplish their roles in differentiation, development, and cancer biology.
My dissertation focused specifically on CDK4/6 inhibitors. As with many chemotherapies, these agents work incredibly well for some people but not others.
I uncovered a new cell cycle transition called senescence after growth arrest (SAGA). Senescence is the state in which cells stop dividing but continue communicating with their neighbors. Using cellular and molecular approaches to study the mechanism that underlies this transition, I found that a number of key proteins are required for SAGA to take place in cells. These proteins were also differentially expressed in people treated with CDK4/6 inhibitors.
This exciting finding suggests that SAGA is an important clinical consideration for treatment with CDK4/6 inhibitors. An improved understanding of this pathway may be used in the future to predict whether people will respond to the therapy and to help develop combination therapies, both of which have the potential to improve patient outcomes.
GSK and Dr. Koff taught me project management, critical thinking, problem-solving, and communication skills that have helped me grow as a scientist and propel my professional career. Despite my love of bench science, the clinical exposure I gained at GSK broadened my career interests to pursue the clinical side of research. I am now a clinical research coordinator at the University of Pennsylvania.

All living things are defined by the genetic material called DNA. It is critical to maintain the integrity of the genetic information encoded by DNA. When the quality of DNA is compromised, diseases can arise.
I completed my dissertation research under the mentorship of developmental biologist Maria Jasin. Her lab focuses on how a cell guards its DNA against damage and how abnormalities in this process can lead to cancer. My thesis aimed to better understand BRCA2, an important guardian of DNA integrity.
People carrying mutations in BRCA2 at birth have an increased risk of developing breast cancer and ovarian cancer. However, in contrast to the accelerated cell proliferation that is the hallmark of cancer, BRCA2 mutations can lead to cell death. It is also unclear which BRCA2 function is key to maintaining the health of breast cells.
To shed light on these mysteries, I developed a model for BRCA2 mutations in normal human breast cells. My research delineated for the first time the consequences of BRCA2 inactivation in a physiological and disease-related context. The work uncovered how cell death is triggered and, importantly, how this process can be bypassed, which may be a basis for tumor formation.
The model I built also allowed me to dissect each individual function of BRCA2 in normal breast cells. This information, together with what we learned from previous studies in cancer cells, revealed that different cells rely on different BRCA2 functions to maintain DNA integrity and support cell survival.
Overall, my thesis study uncovered how cells carrying BRCA2 mutations die and provided insight into how cancer can arise in the face of these aberrations. It also suggested potential weaknesses in BRCA2-mutant cells that can be exploited as novel therapeutic strategies.
I am now a research fellow in the laboratory of physician-scientist Charles Sawyers, Chair of the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering.
I enrolled in GSK as part of the Tri-Institutional MD-PhD Program, a physician-scientist training program jointly sponsored by Weill Cornell Medicine, The Rockefeller University, and Memorial Sloan Kettering. The scientific environment at Memorial Sloan Kettering is exceptional for such collaborative, translational research, and GSK’s core course is well designed to introduce students to the spectrum of work being done at MSK.
I’m generally interested in better understanding the biological mechanisms of disease in order to facilitate the design of more-rational treatment regimens. I completed my thesis work under the mentorship of SKI Director Joan Massagué, whose research focuses on TGFβ signaling and how cancer metastasizes, or spreads.
TGFβ is a signal that cells use to communicate with one another. When I first learned about TGFβ signaling in my undergraduate courses, I was intrigued by the seeming paradox that it can either promote tumor growth or suppress it. How does one signal achieve such different results?
I studied this question in pancreatic ductal adenocarcinoma (PDA), a disease in which the TGFβ pathway is genetically silenced in half of tumors. My goal was to understand how TGFβ signaling is tumor suppressive, which leads to its silencing in PDA. I also sought to determine how tumors that do not genetically modify the pathway survive the tumor suppression imposed by TGFβ.
Using a combination of bioinformatics analysis, cell lines, organoids, mouse models, and clinical samples, I found that TGFβ changes the expression of key transcriptional regulators in pancreatic cells. In cells that have begun to accumulate oncogenic mutations, this usually leads to cell death. However, the effect of TGFβ on some of those key transcriptional regulators is altered in cells that receive certain signals from the tumor microenvironment or develop additional genetic alterations. This allows those cells to survive in the presence of TGFβ and form tumors.
Our work shed light on the mechanisms by which TGFβ cooperates with multiple signaling pathways to promote PDA growth. The findings also uncovered transcriptional regulators that are key to PDA progression. In doing so, we identified potential new targets in PDA, which is currently the fourth leading cause of cancer deaths in the United States.
The Massagué lab, GSK, and MSK have provided wonderful opportunities for me to learn from the best in cancer biology and translational research. I am now finishing the medical phase of my MD-PhD at Weill Cornell Medical School.

As a participant in GSK’s Summer Undergraduate Research Program, I spent ten weeks working in the laboratory of molecular biologist Ken Marians. Impressed with the welcoming environment and the variety of labs researching different biological problems, I decided to pursue my doctorate at GSK.
I completed my dissertation with the support and mentorship of molecular biologist Scott Keeney. His lab studies the mechanisms by which cells make and repair breaks in their DNA, especially during meiosis — the complex cell-division process that gives rise to reproductive cells, such as eggs and sperm.
Cyclins and cyclin-dependent kinases are the main drivers of the cell cycle. My thesis aimed to uncover if and how an enigmatic cyclin called cyclin B3 contributes to meiosis, spermatogenesis, and oogenesis.
I employed CRISPR-Cas9, a gene-editing tool, to generate a cyclin B3 knockout allele in mice. Our research showed that male mice lacking cyclin B3 were completely normal. They did not need cyclin B3 to complete meiotic division and produce sperm cells. This was surprising because, in mammals, cyclin B3 expresses in germ cells but not in somatic cells.
However, female mice lacking cyclin B3 could not produce any progeny. In collaboration with Katja Wassmann’s developmental biology laboratory at the Institut de Biologie, Paris Seine, we found that cyclin B3 was required for oocytes to complete their first division. Moreover, once we added cyclin B3 from other organisms, such as frogs or zebrafish, meiotic division was restored, indicating an evolutionarily conserved role for this cyclin.
Although cyclin B3 is not expressed in normal somatic cells, others have shown that the cyclin B3 gene is fused with an oncogene called BCOR in certain sarcomas. Understanding the role of cyclin B3 in healthy cells might provide important clues as to how it may contribute to the development of sarcoma.
Overall, these findings are important because it is the first time that the role of cyclin B3 has been addressed in mammals. However, more research is needed to uncover the mechanistic details of how it drives the first meiotic division.
After graduation, I will join the Swiss Federal Institute of Technology, where I will utilize CRISPR-Cas9-mediated genome engineering to understand novel genetic pathways in response to DNA damage in the cells.

I decided to pursue my doctorate at GSK because of the breadth and quality of the research at MSK.
I completed my dissertation research in the laboratory of biologist Scott Lowe, Chair of the Cancer Biology and Genetics Program at SKI. His lab investigates how tumor-suppressor genes work to block the formation of cancer and studies the genetic drivers that cancer depends on to thrive. Dr. Lowe takes on ambitious projects, and I was excited to pursue some of these biological questions in his lab.
My thesis focused on fibrolamellar hepatocellular carcinoma (FL‐HCC). We generated a model of this rare form of liver cancer, which afflicts adolescents and young adults and has limited effective treatment options. Although the cause of FL‐HCC is unknown, a genetic fusion between DNAJB1 and PRKACA occurs in the liver of these patients during childhood.
We used genome-engineering tools, including CRISPR, to recreate this observed genetic fusion in mice and cause tumors that resemble the human disease. Using animal models accelerates discovery and enables researchers to perform controlled experiments before exposing people to new medicines in clinical trials. The platform we generated has helped us better understand FL‐HCC development.
I was also interested in cancer as an evolutionary process. Like ecosystems, cancers with high levels of diversity may be more resilient. This is particularly important to understand in the context of cancer that recurs after treatment. Our studies found that tumors caused by mutations in p53, the most commonly mutated tumor-suppressor gene, are more diverse and resilient than those in which p53 remains intact. More research is needed to understand this fascinating and critical process.
My training at GSK has helped me grow as a scientist. I am now studying cancer metabolism as a postdoctoral fellow at Weill Cornell Medical College.

I majored in chemistry as an undergraduate but soon found myself growing more interested in human health. My decision to come to GSK was based on my desire to work in cancer research after being treated for soft tissue sarcoma when I was 20.
I completed my dissertation research in the laboratory of chemical biologist Minkui Luo. I liked that the lab sits at the interface of chemistry and biology and afforded me the opportunity to work from both perspectives, using chemistry tools to address questions in biology.
Dr. Luo’s lab uses chemical biology methods to study a class of enzymes called protein methyltransferases, which are known to be essential for mammalian development and survival and are promising targets for new cancer drugs. Protein methyltransferases modify other proteins by placing methyl groups on their amino acid side chains. These methylations are important for the function of the modified proteins but difficult to detect and study due to their small size.
The lab team developed a chemical biology platform to circumvent this difficulty. The platform helped us identify the methyltransferase substrates of interest and learn more about their biological role.
My dissertation work focused on a subtype of protein methyltransferases known as arginine methyltransferases, which have substrate scopes that are poorly understood. I used the lab’s chemical biology platform to study the essential human methyltransferase PRMT5 and a yeast enzyme called Hmt1, which is highly analogous to an essential human enzyme called PRMT1.
After successfully screening these enzymes to find new substrates, I discovered that both yeast and human methyltransferases modify a group of proteins called translation initiation factors. These factors are necessary to guide mRNA to the ribosome, where it is used as a template for the synthesis of new proteins.
Translation initiation is a fundamental biological process. It was not previously known that protein arginine methyltransferases play a role in its regulation. My thesis demonstrated a novel evolutionarily conserved role for protein arginine methylation in this process. More research is needed to determine the exact biochemical mechanism through which arginine methylation affects translation initiation, but we have determined that it plays an important role.
Although the work doesn’t directly relate to cancer, PRMT5 is a drug target of great interest in many cancer types, most notably mantle cell lymphoma. PRMT5 inhibitors are currently in early stage clinical trials for people with this disease. Work that sheds more light on PRMT5 function may help identify other cancers where PRMT5 inhibitors could be effective.
I’m now a medical writer for WebMD, focusing on continuing medical education for oncologists.

I chose to pursue my graduate training at GSK because of its unique focus on basic and translational science and because of the exposure it provides to outstanding faculty and state-of-the-art research facilities. I completed my dissertation under the guidance of developmental biologist Danwei Huangfu, a visionary and insightful researcher. Her lab uses human embryonic stem cells to model pancreatic development and disease.
Dr. Huangfu’s mentorship has helped me become a better scientist. I am particularly interested in regenerative medicine. My thesis project centered on the identification of a novel regulator of endoderm lineage differentiation. Endoderm cells are the progenitor cells of the pancreas. I used the gene-editing tool CRISPR to search for genes that play a role in either promoting or inhibiting endoderm lineage differentiation.
My dissertation research identified the JNK-JUN signaling pathway as a negative regulator of endoderm differentiation. We demonstrated proof of principle that using a JNK inhibitor to hinder this pathway promoted endoderm lineage differentiation to progenitor lineage cells in the pancreas and lungs. Improving the efficiency of lineage differentiation will help in the development of cell replacement therapies. I am proud to note that we have been granted a patent for this work.
In addition, we collaborated with Michael Beer at Johns Hopkins University on mechanism studies that found that the AP-1 DNA motif is enriched when JNK signaling is activated in human embryonic stem cells. AP-1 motifs are also enriched in Kras-mutant cancers, such as squamous cell carcinoma. The next step in this research is to test whether a JNK inhibitor can be used to reduce the abundance of the AP-1 motif in other cell types to exit its current lineage identity and promote differentiation.
I am now a scientist at CRISPR Therapeutics, where I am using regenerative medicine to develop novel cell and gene therapies.

I chose to pursue my PhD at GSK because I was interested in research opportunities related to immunotherapy that could translate to patient care. I conducted my dissertation research under the mentorship of physician-scientist Nai-Kong Cheung. His laboratory focuses on engineering antibodies and using immune cells to treat pediatric cancers. I was also mentored by immunologist Morgan Huse, whose lab studies the structure and function of immune-cell-to-cell interactions.
Antibodies are produced by the immune system to fight infection. They can also be engineered to treat many different cancer types. A bispecific antibody (BsAb) is an engineered antibody that contains two antigen-binding sites in one molecule. There has been increasing interest in using BsAb for diagnostic and therapeutic purposes.
Although there are more than 60 classes of BsAbs in development, the relative significance of the features needed to generate them is largely unknown. My thesis work focused on T cell BsAbs, which bind to both T cells and tumor cells. I sought to identify the design parameters — including structural, biophysical, and functional properties — that significantly enhance BsAbs’ potency and influence their function in vitro and in vivo.
I undertook a detailed evaluation of an extensive array of structural variants to arrive at the ideal design to target polyclonal T cells in human tumors. The results highlight the importance of affinity, valency, and spatial configuration as important parameters needed to drive strong BsAb-mediated T cell responses against antigen-expressing tumors. Our work provides critical guidelines for improving BsAb function in the design of next-generation immunotherapies.
As part of my PhD training, I also collaborated with nuclear medicine physician Steven Larson and senior research scientist Sarah Cheal in the Molecular Pharmacology Program at SKI. I helped develop a novel platform for pretargeted radioimmunotherapy (PRIT) to deliver massive doses of radiation to mice bearing human tumors. The new platform reduces the existing PRIT approach from three steps to two — an improvement that aims to simplify clinical translation and reduce toxicity.
I am now a postdoctoral research fellow at MSK, studying immunology and antibody engineering for the development of novel therapeutics.

I chose to earn my graduate degree at GSK because of its strong focus on biology, its ability to bridge clinical research and basic science, and its innovative approach to classes and rotations. I completed my thesis work under the guidance of cancer biologist and oncologist Richard White. His lab uses zebrafish to dissect interactions between tumor cells and the microenvironment that promote tumor progression and metastasis.
My dissertation focused on the role of error-prone DNA polymerases and a process called stress-induced mutagenesis (SIM) in melanoma progression and drug resistance.
Activation of the MAPK pathway is central to melanoma. However, the effectiveness of drugs that inhibit this pathway has been limited because resistance quickly develops. Previous work in bacteria has shown that resistance can develop when an error-prone DNA polymerase is activated during SIM. Error-prone DNA polymerases are more likely to insert the wrong base while replicating DNA, which can result in mutations that give a cell an advantage.
Therefore, SIM provides a mechanism through which a cell can temporarily increase its mutation rate during stressful conditions, such as exposure to a drug, in order to acquire an advantageous mutation that causes it to become resistant to that drug. My thesis examined whether a similar process occurs in human cancer.
To demonstrate that cancer cells could activate the error-prone DNA polymerase kappa (polκ), we treated melanoma cell lines with a variety of drugs and noted the effects on the level of polκ expression as well as its location in the cells.
We observed that treatment of melanoma cell lines with MAPK inhibitors led to both an increase in polκ mRNA levels as well as a shift of polκ protein from the cytoplasm into the nucleus. Similar results were observed for other forms of stress, such as glucose starvation. Furthermore, we discovered that the mTOR signaling pathway and the nuclear export machinery both played a central role in the shift of the polκ protein.
We also studied the functional consequences of having a high level of polκ in the nucleus. Experiments in zebrafish demonstrated that overexpression of polκ accelerated melanoma formation. Studies in human cell lines showed that overexpression of polκ can result in increased drug resistance.
Our findings provide important insights into tumor evolution that could be translated into new approaches for reducing the risk of developing resistance to cancer treatment.
I am now a postdoctoral research fellow studying Ewing sarcoma in Elizabeth Lawlor’s lab in the Department of Pediatrics at the University of Michigan Medical School.

I chose to pursue my graduate degree at GSK because of the rigorous science being conducted at MSK.
I completed my dissertation in the laboratory of Stephen Long. He uses cutting-edge techniques to understand the structure and function of ion channels, the electrical hardware of living organisms. This fascinating class of proteins first captivated me during my undergraduate studies in the United Kingdom. By controlling the flow of charged atoms across cell membranes, ion channels play vital roles in diverse processes, from vision and touch to heart flow and motor function.
When I joined Dr. Long’s lab, I was tasked with trying to learn more about a particular ion channel called bestrophin. Bestrophin is a chloride channel located in the retina of the eye. Mutations in this protein cause blindness. A high-resolution structure of bestrophin had been recently determined by others in Dr. Long’s lab, but little was known about how this channel actually worked.
My thesis sought to understand the fundamental mechanisms of bestrophin channel function by using a combination of structural and functional approaches, including X-ray crystallography, electrophysiology, and single-particle cryo-electron microscopy. My research laid out the mechanisms of ion selectivity and calcium-dependent activation of the bestrophin channel. We now have a much clearer understanding of the structure of this ion channel, including which parts are responsible for its characteristic behaviors and why some mutations can lead to disease.
I am now further pursuing my interest in ion channels as a postdoctoral researcher at The Rockefeller University. I hope to uncover more about the molecular basis of touch and other pressure-sensing biological processes.

I chose to pursue my doctoral degree at GSK because of its reputation as being on the cutting-edge of cancer research. I completed my dissertation in the laboratory of cancer biologist Andrea Ventura. I was drawn to his work studying noncoding RNAs, especially miRNAs, and their contribution to the development of cancer.
Gene fusions — such as genetic deletions, inversions, and translocations — help fuel the development of cancer. A number of cancer drugs have been developed to target these fusions.
New gene-editing technologies have enabled researchers to model gene fusions that have been traditionally hard to model in a physiological manner. Building better mouse models with these tools allows us to study a variety of tumors driven by gene fusions and gain a fuller understanding of cancer fusion biology.
With the advent of the gene-editing tool CRISPR, we can now faithfully recapitulate the genetic events that lead to the formation of gene fusions. We can also examine other factors involved in tumor genesis, such as the loss of copy number of wild type alleles.
My dissertation research sought to model the interchromosomal translocation between NPM1 and ALK. This fusion is found in anaplastic large-cell lymphoma. I developed a viral vector and used CRISPR-Cas9 to model NPM1-ALK in murine hematopoietic cells. I showed that NPM1-ALK generated by CRISPR was able to transform hematopoietic cells in an allogeneic transplantation model.
I appreciate Dr. Ventura’s support and the lessons he imparted, including how to cope with the pressures of academic research and be resilient in the face of roadblocks. These skills, together with my dissertation work involving CRISPR, have fueled my interest in using gene technologies to develop new therapies. I am now a healthcare equity research associate at Landenburg Thalmann.