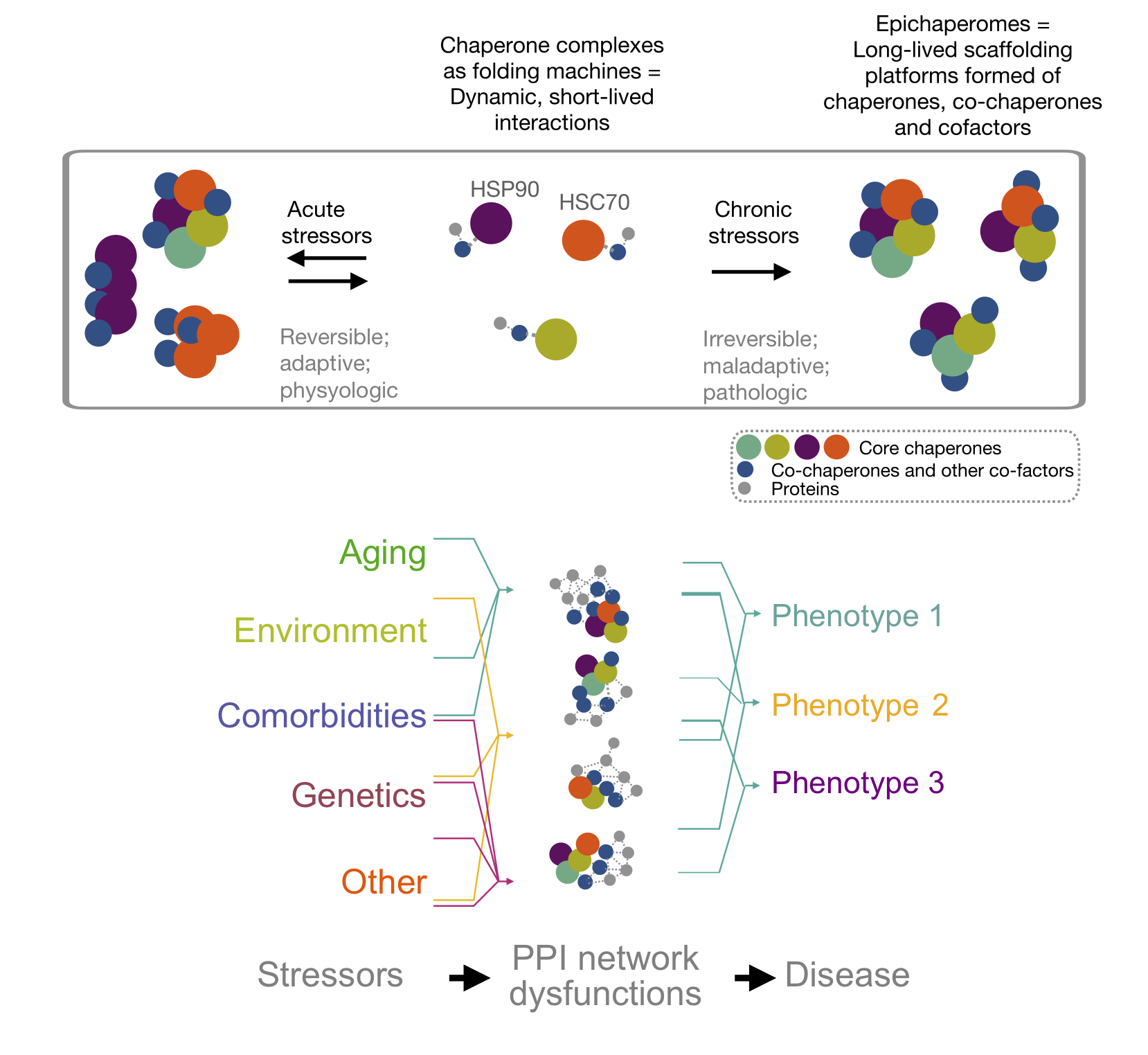
Chiosis G, Digwal CS, Trepel JB, Neckers L. Structural and functional complexity of HSP90 in cellular homeostasis and disease. Nat Rev Mol Cell Biol. 2023. doi: 10.1038/s41580-023-00640-9. https://pubmed.ncbi.nlm.nih.gov/37524848/
Ginsberg SD, Sharma S, Norton L, Chiosis G. Targeting stressor-induced dysfunctions in protein-protein interaction networks via epichaperomes. Trends Pharmacol Sci. 2023;44(1):20-33. doi: 10.1016/j.tips.2022.10.006. https://pubmed.ncbi.nlm.nih.gov/36414432/
Rodina A, Xu C, Digwal CS, Joshi S, Patel Y, Santhaseela AR, Bay S, Merugu S, Alam A, Yan P, Yang C, Roychowdhury T, Panchal P, Shrestha L, Kang Y, Sharma S, Almodovar J, Corben A, Alpaugh ML, Modi S, Guzman ML, Fei T, Taldone T, Ginsberg SD, Erdjument-Bromage H, Neubert TA, Manova-Todorova K, Tsou MB, Young JC, Wang T, Chiosis G. Systems-level analyses of protein-protein interaction network dysfunctions via epichaperomics identify cancer-specific mechanisms of stress adaptation. Nat Commun. 2023;14(1):3742. doi: 10.1038/s41467-023-39241-7. https://www.nature.com/articles/s41467-023-39241-7
Castelli M, Yan P, Rodina A, Digwal CS, Panchal P, Chiosis G, Moroni E, Colombo G. How aberrant N-glycosylation can alter protein functionality and ligand binding: An atomistic view. Structure. 2023. doi: 10.1016/j.str.2023.05.017. https://pubmed.ncbi.nlm.nih.gov/37343552/
Bolaender A, He H, Joshi S, Sharma S, Digwal CS, Patel HJ, Sun W, Imber BS, Ochiana SO, Patel MR, Shrestha L, Shah SK, Wang S, Karimov R, Tao H, Patel PD, Martin AR, Yan P, Panchal P, Almodovar J, Corben A, Rimner A, Ginsberg SD, Lyashchenko S, Burnazi E, Ku A, Kalidindi T, Lee SG, Grkovski M, Beattie BJ, Zanzonico P, Lewis JS, Larson S, Rodina A, Pillarsetty N, Tabar V, Dunphy MP, Taldone T, Shimizu F, Chiosis G. Chemical tools for epichaperome-mediated interactome dysfunctions of the central nervous system. Nat Commun. 2021;12(1):4669. doi: 10.1038/s41467-021-24821-2. https://pubmed.ncbi.nlm.nih.gov/34344873/
Ginsberg SD, Joshi S, Sharma S, Guzman G, Wang T, Arancio O, Chiosis G. The penalty of stress - Epichaperomes negatively reshaping the brain in neurodegenerative disorders. J Neurochem. 2021;159(6):958-79. doi: 10.1111/jnc.15525. https://pubmed.ncbi.nlm.nih.gov/34657288/
Joshi S, Gomes ED, Wang T, Corben A, Taldone T, Gandu S, Xu C, Sharma S, Buddaseth S, Yan P, Chan LYL, Gokce A, Rajasekhar VK, Shrestha L, Panchal P, Almodovar J, Digwal C, Rodina A, Pillarsetty N-K, Miclea V, Peter RI, Ginsberg SD, Tang L, Mattar M, de Stanchina E, Yu KH, Lowery M, Grbovic-Huezo O, O’Reilly EM, Janjigian Y, Healey JH, Jarnagin WR, Allen PJ, Sander C, Erdjument-Bromage H, Neubert TA, Leach SD, Chiosis G. Pharmacologically controlling protein-protein interactions through epichaperomes for therapeutic vulnerability in cancer. Commun Biol. 2021;4(1):1333. doi: 10.1038/s42003-021-02842-3. https://pubmed.ncbi.nlm.nih.gov/34824367/
Inda MC, Joshi S, Wang T, Bolaender A, Gandu S, Koren Iii J, Che AY, Taldone T, Yan P, Sun W, Uddin M, Panchal P, Riolo M, Shah S, Barlas A, Xu K, Chan LYL, Gruzinova A, Kishinevsky S, Studer L, Fossati V, Noggle SA, White JR, de Stanchina E, Sequeira S, Anthoney KH, Steele JW, Manova-Todorova K, Patil S, Dunphy MP, Pillarsetty N, Pereira AC, Erdjument-Bromage H, Neubert TA, Rodina A, Ginsberg SD, De Marco Garcia N, Luo W, Chiosis G. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat Commun. 2020 Jan 16;11(1):319. doi: 10.1038/s41467-019-14082-5.
Yan P, Patel HJ, Sharma S, Corben A, Wang T, Panchal P, Yang C, Sun W, Araujo TL, Rodina A, Joshi S, Robzyk K, Gandu S, White JR, de Stanchina E, Modi S, Janjigian YY, Hill EG, Liu B, Erdjument-Bromage H, Neubert TA, Que NLS, Li Z, Gewirth DT, Taldone T, Chiosis G. Molecular Stressors Engender Protein Connectivity Dysfunction through Aberrant N-Glycosylation of a Chaperone. Cell Rep. 2020 Jun 30;31(13):107840. doi: 10.1016/j.celrep.2020.107840.
Rodina A, Wang T, Yan P, Gomes ED, Dunphy MP, Pillarsetty N, Koren J, Gerecitano JF, Taldone T, Zong H, Caldas-Lopes E, Alpaugh M, Corben A, Riolo M, Beattie B, Pressl C, Peter RI, Xu C, Trondl R, Patel HJ, Shimizu F, Bolaender A, Yang C, Panchal P, Farooq MF, Kishinevsky S, Modi S, Lin O, Chu F, Patil S, Erdjument-Bromage H, Zanzonico P, Hudis C, Studer L, Roboz GJ, Cesarman E, Cerchietti L, Levine R, Melnick A, Larson SM, Lewis JS, Guzman ML, Chiosis G. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature. 2016;538(7625):397-401. PMCID: PMC5283383. Altmetric 439 PubMed
Kishinevsky S, Wang T, Rodina A, Chung SY, Xu C, Philip J, Taldone T, Joshi S, Alpaugh ML, Bolaender A, Gutbier S, Sandhu D, Fattahi F, Zimmer B, Shah SK, Chang E, Inda C, Koren J, Saurat NG, Leist M, Gross SS, Seshan VE, Klein C, Tomishima MJ, Erdjument-Bromage H, Neubert TA, Henrickson RC, Chiosis G, Studer L. HSP90-incorporating chaperome networks as biosensor for disease-related pathways in patient-specific midbrain dopamine neurons. Nature Communications. 2018;9(1):4345.
Kourtis N, Lazaris C, Hockemeyer K, Balandran JC, Jimenez AR, Mullenders J, Gong Y, Trimarchi T, Bhatt K, Hu H, Shrestha L, Ambesi-Impiombato A, Kelliher M, Paietta E, Chiosis G, Guzman ML, Ferrando AA, Tsirigos A, Aifantis I. Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat Med. 2018;24(8):1157-66. PMCID: PMC6082694. Altmetric 462. PubMed
Zong H, Gozman A, Caldas-Lopes E, Taldone T, Sturgill E, Brennan S, Ochiana SO, Gomes-DaGama EM, Sen S, Rodina A, Koren J 3rd, Becker MW, Rudin CM, Melnick A, Levine RL, Roboz GJ, Nimer SD, Chiosis G, Guzman ML. A Hyperactive Signalosome in Acute Myeloid Leukemia Drives Addiction to a Tumor-Specific Hsp90 Species. Cell Rep. 2015 Dec 15;13(10):2159-73. doi: 10.1016/j.celrep.2015.10.073. Epub 2015 Nov 25. [PMID: 26628369]
Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N, Caldas-Lopes E, Ross KN, Gonen M, Gozman A, Ahn JH, Rodina A, Ouerfelli O, Yang G, Hedvat C, Bradner JE, Chiosis G, Levine RL. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120(10):3578-93. PMCID: PMC2947224. PubMed
Cerchietti LC, Hatzi K, Caldas-Lopes E, Yang SN, Figueroa ME, Morin RD, Hirst M, Mendez L, Shaknovich R, Cole PA, Bhalla K, Gascoyne RD, Marra M, Chiosis G, Melnick A. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest. 2010;120(12):4569-82. PMCID: PMC2993589. PubMed
Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, Liu F, Saunders LM, Mullally A, Abdel-Wahab O, Leung L, Weinstein A, Marubayashi S, Goel A, Gonen M, Estrov Z, Ebert BL, Chiosis G, Nimer SD, Bernstein BE, Verstovsek S, Levine RL. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489(7414):155-9. PMCID: PMC3991463. PubMed
Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, Que NS, Taldone T, Finotti P, Stephani RA, Gewirth DT, Chiosis G. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9(11):677-84. PMCID: PMC3982621. PubMed
Nayar U, Lu P, Goldstein RL, Vider J, Ballon G, Rodina A, Taldone T, Erdjument-Bromage H, Chomet M, Blasberg R, Melnick A, Cerchietti L, Chiosis G, Wang YL, Cesarman E. Targeting the Hsp90-associated viral oncoproteome in gammaherpesvirus-associated malignancies. Blood. 2013;122(16):2837-47. PMCID: PMC3798998. PubMed
Ambati SR, Lopes EC, Kosugi K, Mony U, Zehir A, Shah SK, Taldone T, Moreira AL, Meyers PA, Chiosis G, Moore MA. Pre-clinical efficacy of PU-H71, a novel HSP90 inhibitor, alone and in combination with bortezomib in Ewing sarcoma. Mol Oncol. 2014;8(2):323-36. PMCID: PMC3982393. PubMed
Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, Qi J, Kleppe M, Patel HJ, Shah SK, Taldone T, Bradner JE, Chiosis G, Levine RL. Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood. 2014;123(13):2075-83. PMCID: PMC3968390. PubMed
Rampal R, Ahn J, Abdel-Wahab O, Nahas M, Wang K, Lipson D, Otto GA, Yelensky R, Hricik T, McKenney AS, Chiosis G, Chung YR, Pandey S, van den Brink MR, Armstrong SA, Dogan A, Intlekofer A, Manshouri T, Park CY, Verstovsek S, Rapaport F, Stephens PJ, Miller VA, Levine RL. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111(50):E5401-10. PMCID: PMC4273376. PubMed
Kucine N, Marubayashi S, Bhagwat N, Papalexi E, Koppikar P, Sanchez Martin M, Dong L, Tallman MS, Paietta E, Wang K, He J, Lipson D, Stephens P, Miller V, Rowe JM, Teruya-Feldstein J, Mullighan CG, Ferrando AA, Krivtsov A, Armstrong S, Leung L, Ochiana SO, Chiosis G, Levine RL, Kleppe M. Tumor-specific HSP90 inhibition as a therapeutic approach in JAK-mutant acute lymphoblastic leukemias. Blood. 2015;126(22):2479-83. PMCID: PMC4661170. PubMed
Goldstein RL, Yang SN, Taldone T, Chang B, Gerecitano J, Elenitoba-Johnson K, Shaknovich R, Tam W, Leonard JP, Chiosis G, Cerchietti L, Melnick A. Pharmacoproteomics identifies combinatorial therapy targets for diffuse large B cell lymphoma. J Clin Invest. 2015;125(12):4559-71. PMCID: PMC4665772. PubMed
Culjkovic-Kraljacic B, Fernando TM, Marullo R, Calvo-Vidal N, Verma A, Yang S, Tabbo F, Gaudiano M, Zahreddine H, Goldstein RL, Patel J, Taldone T, Chiosis G, Ladetto M, Ghione P, Machiorlatti R, Elemento O, Inghirami G, Melnick A, Borden KL, Cerchietti L. Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B-cell lymphomas. Blood. 2016;127(7):858-68. PMCID: PMC4760090. PubMed
Guo A, Lu P, Lee J, Zhen C, Chiosis G, Wang YL. HSP90 stabilizes B-cell receptor kinases in a multi-client interactome: PU-H71 induces CLL apoptosis in a cytoprotective microenvironment. Oncogene. 2017;36(24):3441-9. PMCID: PMC5645670. PubMed
A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Cerchietti LC, Lopes EC, Yang SN, Hatzi K, Bunting KL, Tsikitas LA, Mallik A, Robles AI, Walling J, Varticovski L, Shaknovich R, Bhalla KN, Chiosis G, Melnick A. Nat Med. 2009 Dec;15(12):1369-76.
Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A, Guo Y, Wu N, de Stanchina E, White J, Gross SS, Ma Y, Varticovski L, Melnick A, Chiosis G.Proc Natl Acad Sci U S A. 2009 May 19;106(20):8368-73.