Scientist Gabriela Chiosis studies how proteins take shape and interact with each other.
Sloan Kettering Institute scientist Gabriela Chiosis, a member of the Chemical Biology Program, has devoted her research to studying how proteins take shape and interact with each other and how it can lead to disease when these proteins go awry.
On August 3, 2021, she published her latest paper in Nature Communications, which demonstrated how to discover and develop a new drug that has the ability to detect and reverse protein malfunctions in the central nervous system. The drug is already being tested in a clinical trial for Alzheimer’s disease, and another trial will launch soon for glioblastoma, the most aggressive type of brain cancer.
Here, Dr. Chiosis speaks about her research and where it’s heading.
How is your research different from what many other scientists at Memorial Sloan Kettering Cancer Center are studying?
When most people think about the causes of cancer, they think about genetic changes that lead to disease. MSK has a large effort based on tumor sequencing and assigning treatments based on genetic mutations in tumors.
I think of gene mutations as just one stressor that can affect the way cells behave. Many other kinds of changes in a cell and its environment also can impact its function. These changes can accumulate over a person’s lifetime, eventually leading to proteins that misinteract. This in turn has a negative effect on cell behavior.
When you talk about misinteracting proteins, what does that mean?
Ensuring that the thousands of proteins located inside a cell interact properly is important for keeping that cell functioning normally. Chaperones are proteins that help proteins in normal cells fold and come together in the correct way, making sure that they interact correctly with the correct partner.
In 2016, I published a paper that reported how chaperones band together into disease-enabling networks, which we named epichaperomes. In cancer cells, the proteins that improperly come together are involved in cellular processes needed for a cancer cell to survive. Consequently, we believe that targeting these epichaperomes with drugs may be a good way to destroy cancer cells, because it takes away their survival mechanism.
In neurodegenerative diseases like Alzheimer’s, on the other hand, the proteins that misinteract are the ones that are key to the connections that are important for memory and thinking. We published this finding last year. In this case, targeting the epichaperome is actually restorative, because it takes away the mechanism that made the nerve cell defective in the first place.
What was discovered about drugs that can target epichaperomes in this paper?
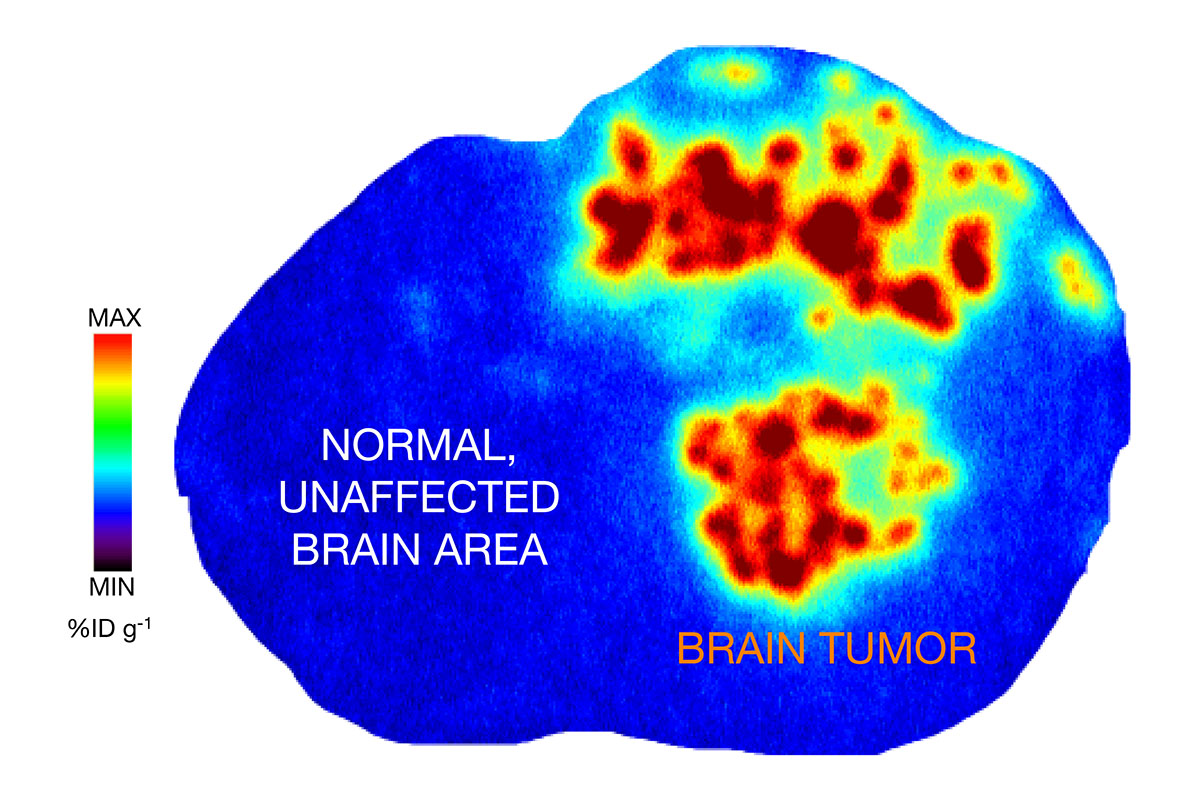
A brain scan shows PU-AD localized to a tumor in the brain of a mouse. Red, orange, and yellow represent the highest concentrations of the drug. Credit: Adapted from an image in Nature Communications.
There are major challenges to making drugs that target the epichaperome. First, they need to be able to differentiate between normal chaperones, which are found in every cell in the body, and the disease epichaperomes. The initial drug that we developed to do this, called PU-H71, has been studied in clinical trials for lymphoma, breast cancer, and acute myeloid leukemia. But PU-H71 is not able to cross the blood-brain barrier, making it ineffective against brain tumors and Alzheimer’s.
Our second challenge was to create an epichaperome drug that is able to cross this barrier. This led to our discovery of another drug, called PU-AD. Our study from last year showed in mice that PU-AD can break apart the faulty protein networks created by epichaperomes and fix signaling problems between nerve cells. After being given the drug, the mice performed better on memory tests.
Now we are expanding the use of PU-AD to glioblastoma tumors that contain epichaperomes. By adding a radioactive probe, we showed in mice that the drug is going where it’s supposed to go — to the tumor, but not to parts of the animals’ brains or bodies that don’t have epichaperomes. We demonstrated that the drug goes not only to the cancer cells themselves, but to the cells in the microenvironment around the cancer cells. These cells also contribute to tumor survival, including resistance to chemotherapy and radiation.
We believe this type of imaging can help us determine which patients are likely to benefit from drugs targeting the epichaperome. Not all cancers contain epichaperomes or the same levels of epichaperomes, so we expect that these drugs will be used as part of precision oncology treatments and given only to patients whose tumors have the right features.
What’s next for this research?
PU-AD is already in a phase II study for people with mild Alzheimer’s disease. A phase I trial showed that it was safe in healthy volunteers who didn’t have Alzheimer’s. Later this year, we plan to launch a phase I study evaluating the safety of PU-AD for glioblastoma. This multicenter trial will be conducted at MSK and other hospitals.
Who were your collaborators on this study?
Viviane Tabar [Chair of the Department of Neurosurgery and Theresa Feng Chair in Neurosurgery] was an important part of this research. Her group gave us the glioblastoma patient samples that were implanted in the mice and allowed us to test the drug against brain cancer.
MSK’s nuclear medicine and imaging teams, including radiochemists Jason Lewis and Naga Vara Kishore Pillarsetty and nuclear medicine physician Mark Dunphy, also worked on this research, including providing the radioprobes and the technology for detecting them in the brain.
- Stressors that accumulate over a person’s lifetime can eventually lead to proteins that “misinteract,” which results in cell malfunction.
- Networks inside cells called epichaperomes cause proteins to come together improperly and can lead to disease.
- Researchers at MSK are developing drugs that target these epichaperomes and restore normal cell functions.
- These drugs are in clinical trials for Alzheimer’s disease and different types of cancer.