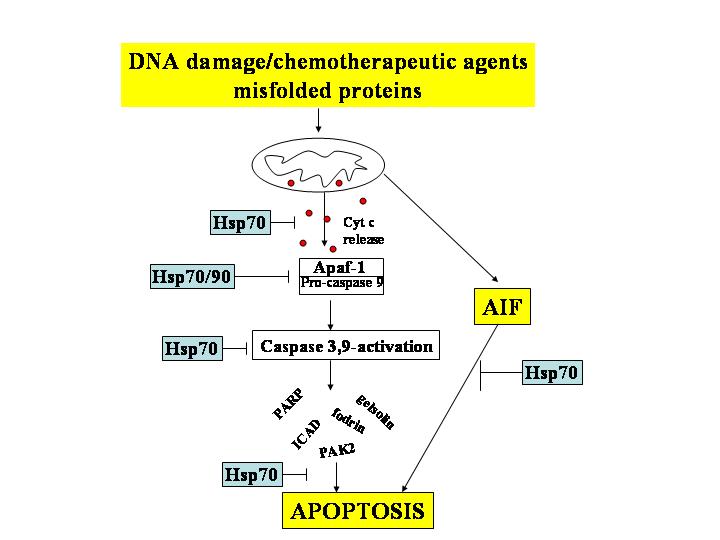
It is documented that inhibition of Hsp70 expression by anti-sense Hsp70 cDNA resulted in inhibition of tumor cell proliferation and induction of apoptosis. Depletion of Hsp70 by Ad.asHsp70 led to massive cell death of all tumorigenic cell lines tested (carcinomas of breast, colon, prostate and liver as well as glioblastoma). In spite of an effective depletion of Hsp70, Ad.asHsp70 had no effect on the survival or growth of fetal fibroblasts or non-tumorigenic epithelial cells of breast or prostate. Inhibition of hsp70 gene expression has been documented after pharmacological intervention with the flavanoid quercetin. The agent induced apoptosis in several tumor cell lines. In addition, inhibition of hsp70 accumulation by quercetin made cells more susceptible to apoptotic inducers. Quercetin also sensitized cells to hyperthermia, chemotherapy and radiation. Inhibition of hsp70 synthesis as well as induction of apoptosis by treatment with quercetin combined with hyperthermia wss reported to be confined to leukemic cells, and not to normal hematopoietic progenitor cells. In spite of its evident utility in cancer treatment, quercetin is not potent enough to grant its clinical use. Since the introduction of anti-sense mRNA or siRNAs into humans will be problematic because the extent of inhibition cannot be modulated, and the effects of quercetin are likely pleiotropic, small molecules that directly compromise but not completely inhibit the activities of Hsp70 chaperones will prove clinically valuable to combat cancer. In addition, the above data suggest that an Hsp70 inhibitor concentration can be identified that will not be toxic to healthy cells. To date, however, Hsp70 inhibitors have not been tested in cell or animal cancer models, and very few Hsp70 inhibitors have been identified.
We are interested in identifying both inhibitors of Hsp70 activity and expression and efforts in this regard are currently underway.