A Robust Linker for Solid Phase Diversity-Oriented Synthesis
The t-butyldiarylsilyl (TBDAS) linker was developed by Drs. Christine DiBlasi and Daniel Macks in our group for solid phase organic synthesis. This robust linker is stable to a variety of reaction conditions, but can be cleaved with relatively mild, chemoselective fluoride reagents to yield alcohol products.
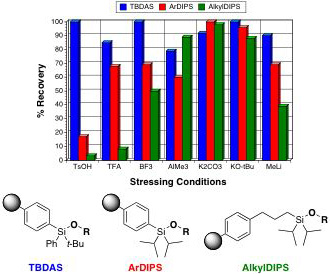
As depicted graphically here, the TBDAS linker (blue) is stable to a range of acidic and basic reaction conditions commonly used in organic synthesis. In contrast, previously reported aryldiisopropylsilyl (ArDIPS, red) and alkyldiisopropylsilyl (AlkylDIPS) linkers are generally less stable to these conditions.
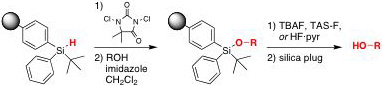
The TBDAS linker is activated by chlorination with dichorodimethylhydantoin, followed by loading of the desired alcohol-containing substrate. After synthetic transformation, the product is cleaved from the solid support by treatment with TBAF, TAS-F, or HF·pyr. The product can be conveniently separated from the cleavage reagents by passage through a short plug of reverse phase over normal phase silica gel.
For full details on the synthesis and use of this linker, see:
DiBlasi, C. M.; Macks, D. E.; Tan, D. S.* “An acid-stable tert-butyldiarylsilyl (TBDAS) linker for solid-phase organic synthesis.” Org. Lett. 2005, 7, 1777-1780.
[ Abstract | PDF | Supporting Info ]
Highlighted in Letters in Organic Chemistry [PDF]