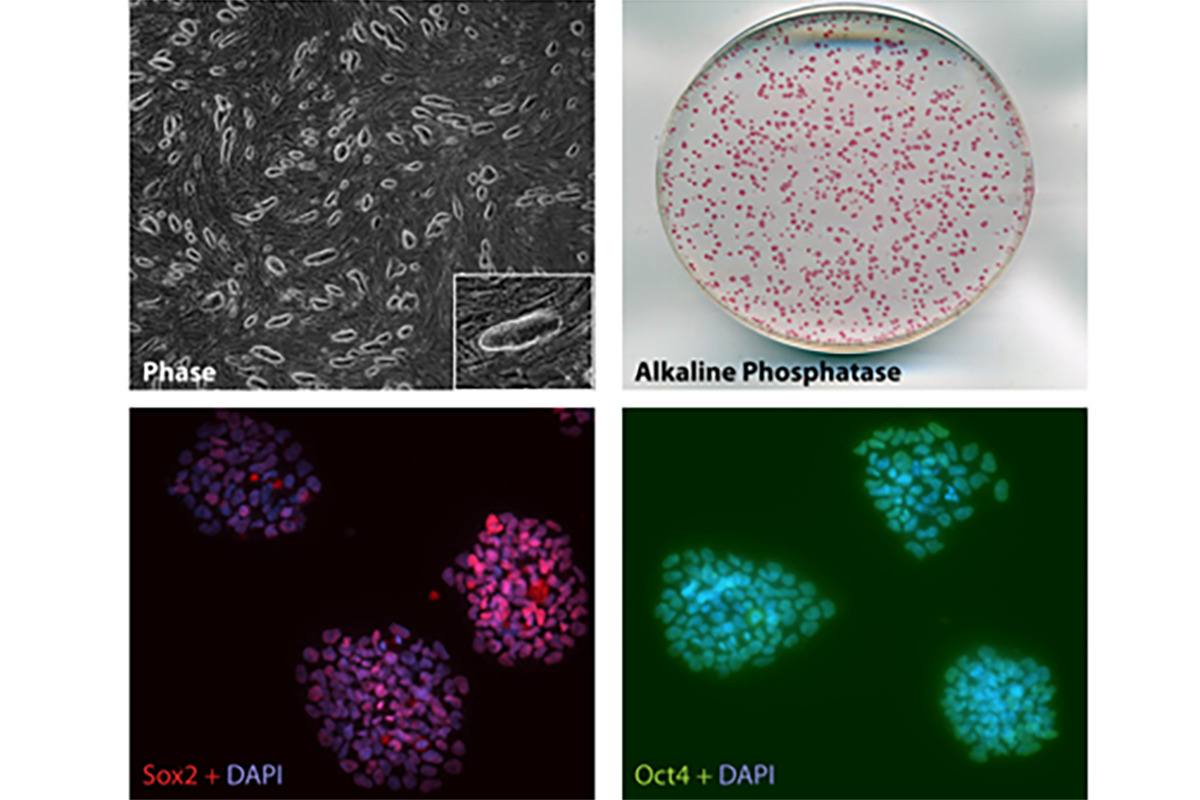
Investigate the establishment of telomere length during development
A major goal of the Sfeir is to understand the mechanism(s) underlying telomere length regulation, which is critical for cellular survival. We are particularly interested in uncovering the pathway(s) that resets telomere length during embryonic development, a key stage that replenished telomere reserves for the adult. The activation of telomerase in the cells of inner cells mass is a crucial step, although its molecular underpinnings are largely unknown. We mapped hTERT enhancers and showed that transcriptional control does not fully account for the difference in hTERT mRNA levels between pluripotent and differentiated cells (Penev et al, biorxiv 2020). Instead, we made a striking observation that telomerase regulation is primarily governed by alternative splicing that involves hTERT exon-2. Furthermore, we implicate the splice co-factor SON in this hTERT splice switch during pluripotency and identify a patient carrying a SON mutation and suffering from dyskeratosis congenita as a result of short telomeres. Altogether, our study reveals that hTERT splicing is key developmental switch for telomerase that can lead to debilitating disease when defective. Our findings hold therapeutic implication both in the context of cancer treatment as well as boosting regenerative capacity of cells.
Telomere dysfunction and cancer
The G-richness and highly repetitive nature of telomeric DNA pose major challenges to the DNA replication machinery. In the lab, we recently highlighted a role for the telomere binding protein, POT1, in assisting the replisome when copying telomere repeats (Pinzaru et al., Cell Reports 2016). Notably, mutations in POT1 have been associated with several types of human cancers, including chronic lymphocytic leukemia (CLL), cutaneous T cell lymphomas (CTCL), and melanoma. We found that perturbations in POT1 (deletions and mutations) triggers telomere replication stress leading to profound telomere dysfunction. Together with the Lazzerini-Denchi lab (NIH), we showed that the loss of POT1 in the common lymphoid progenitor cells (CLPs) promotes the formation of thymic lymphomas in mice. Interestingly, we found that proliferation of cancer cells lacking POT1 is enabled by the attenuation of the ATR kinase pathway, highlighting a novel means by which cancer mutations bypass cell cycle checkpoint. In a subsequent study we interrogated the genetic susceptibilities of cells carrying POT1 mutations and identified a role for the nuclear pore complex in resolving telomere replication defects (Pinzaru et al., Genes & Dev 2020).
A surprising link between telomeres and metabolism
In addition to addressing the role of telomeres in cancer, my lab found that mice lacking the telomere binding protein Rap1 suffer from metabolic dysregulation (Yeung et al., 2013). Rap1 is a major telomere binding protein that also acts as a transcriptional regulator controlling adipogenesis. We highlighted a role for Rap1 in protecting from late-onset obesity. Obesity and its comorbidities are at the heart of public health concern in the 21st century and our work on Rap1 probes a unique link between aging and metabolic diseases.