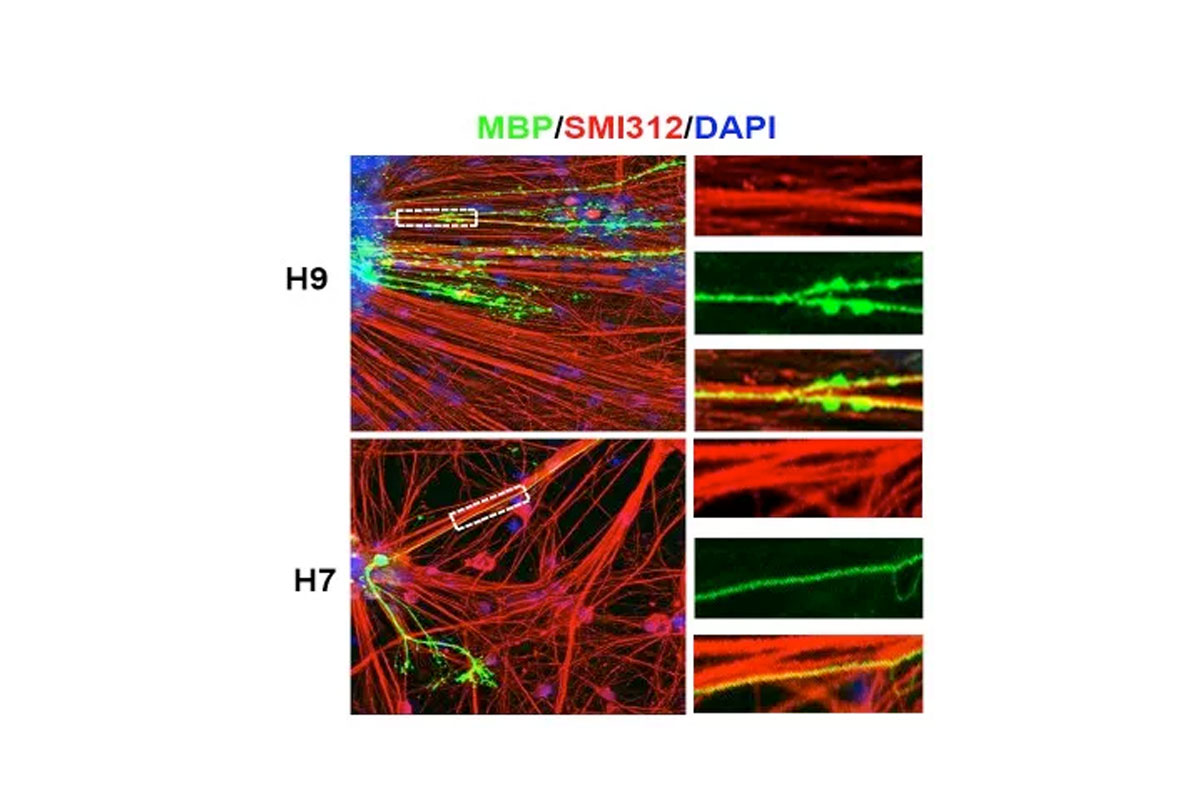
Radiation to the brain is a major contributor to poor quality of life among cancer survivors, yet it remains among the most potent tools in cancer therapy. Whole or partial brain radiation is a cornerstone of the treatment of many brain tumors, primary or metastatic, as well as a major component of prophylactic regimens in the treatment of leukemias. Young children are particularly vulnerable to the side-effects of radiation injury and often receive suboptimal radiation doses as a compromise between efficacy and safety. We have shown that radiation to the brain results in loss or significant depletion of the oligodendrocyte progenitor pool in the brain, both in a rat model we developed as well as in human brain tissue samples. We have also developed a scalable protocol for the efficient derivation of oligodendrocyte progenitors from human ES or iPS cells. The cells are well characterized by phenotype markers, gene expression profile and in vitro myelination. In a major effort over the past several years, we optimized rodent brain radiation to mimic the standard high dose fractionated regimen given to patients (50 Gy). The young rats develop cognitive and motor behavioral deficits within several months of treatment. Analysis of the rat brains shows depletion of the oligodendrocyte progenitor pool as well diffuse demyelination. Grafting resulted in structural repair shown histologically and by electron microscopy, as well as in a graft region-specific behavioral improvement. Forebrain grafts improved cognition while cerebellar grafts were required for amelioration of motor balance. We plan to further optimize the cell culture and in vivo parameters in future pre-clinical studies.