Cancer cells have many ways to protect themselves from the body’s natural defense systems. Recently, researchers in the Sloan Kettering Institute discovered a new one: Some cancer cells are able to form protective droplets called nuclear bodies around the genetic material that makes them cancerous. Now that scientists are aware of this activity, they can begin looking for ways to take aim against it.
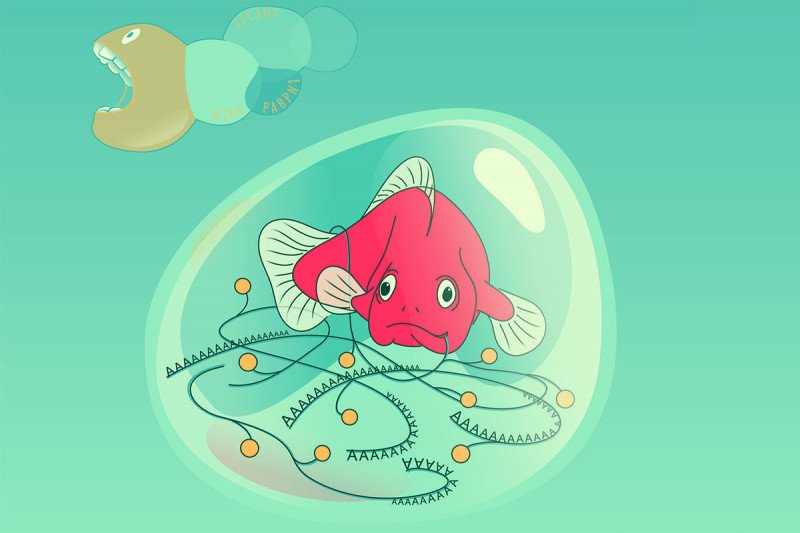
This newly discovered defense mechanism protects messenger RNA (mRNA), which is the molecule that acts as a go-between in the manufacturing of proteins from DNA. The researchers found that when a protein called YTHDC1 binds to mRNA, droplet-like structures are formed to prevent enzymes from breaking down these cancer-causing molecules. When YTHDC1 — and the droplets — are not present, the mRNA is broken down.
“When these molecules get together, they form little droplets that look like what happens when you add balsamic vinegar to olive oil,” says cancer biologist Michael Kharas, a member of the Molecular Pharmacology Program and the study’s senior author. “These droplets appear to be protecting the mRNA from being destroyed.” The findings were published May 27, 2021, in Cancer Cell.
An Unexpected Role in Cancer
Dr. Kharas’s lab focuses on cellular processes related to leukemia. In particular, he studies how acute myeloid leukemia (AML) cells have mRNA that is altered by a process called methylation (m6A), which means that chemicals called methyl groups are attached. This can influence how proteins are made and lead to cancer. His earlier research has shown that a protein called METTL3, which is important for attaching these methyl groups, is required for leukemia growth in animal models. Several biotechnology companies are now developing inhibitors to target this pathway in cancer.
In the new study, his group was again focused on m6A. They wanted to learn more about how it interacts with other proteins in the cell nucleus, including YTHDC1, which they knew was found at high levels in many AML cells. They added YTHDC1 that had been marked with a green fluorescent protein and looked at the cells under the microscope. They were surprised to find that the cells’ nuclei were filled with tiny green dots. Further analysis revealed that these dots were droplets that were protecting the methylated mRNA molecules from enzymes (packaged inside structures called exosomes) that would otherwise break them down.
“These kinds of nuclear structures were identified 20 years ago, but their biophysical properties, what they do, and their role in cancer was completely unexpected,” Dr. Kharas says. “Our research confirms that the number of droplets is much higher in cancer cells than it is in normal cells.”
Dynamic Movement of Droplets in the Cell Nucleus
Fusion of Droplets
A Potential Target for Therapies
Under the microscope, Dr. Kharas and his colleagues could see that the nuclear bodies were dynamic and could move inside the nuclei of the leukemia cells (shown above in the first video). Sometimes the droplets could fuse together and form larger structures (shown above in the second video), but the researchers don’t know what this means. It’s something they plan to study.
“We don’t understand everything about these structures, but our research suggests they are important for certain cancers, including AML,” Dr. Kharas says. “They may provide a potential target for treatment. They also show us a whole new aspect of biology that’s linked to mRNA methylation.”
The co-first author on this paper is Yuanming Cheng, a research scholar in Dr. Kharas’s lab. The other co-first author is Wei Xie, a senior research scientist in the lab of SKI structural biologist Dinshaw Patel. Dr. Patel, a member of SKI’s Structural Biology Program, also contributed to the research.