Scientists from Memorial Sloan Kettering have discovered a unique monoclonal antibody that appears to be very effective at targeting and destroying several types of cancer cells. The research was done in collaboration with the California-based biotechnology company Eureka Therapeutics and was reported March 13 in Science Translational Medicine.
Monoclonal antibodies are molecules that can be engineered to target specific proteins on cancer cells. A number of them are already available to treat a variety of different cancers. The new monoclonal antibody, called ESK1, targets a protein that is associated with many types of cancer and is of great interest to cancer researchers.
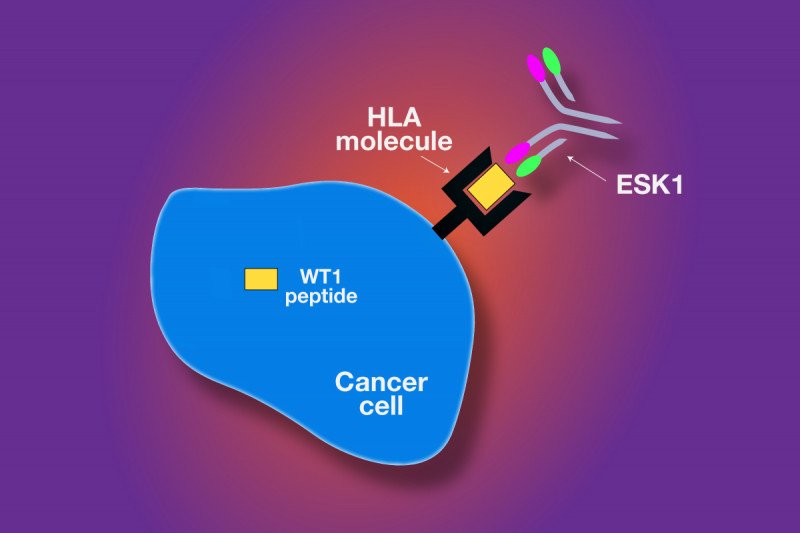
Because of their large size, monoclonal antibodies can target only proteins located on the outside of cancer cells. But ESK1 is different because it is capable of recognizing the presence of a protein that resides within the cell. This is a long-sought goal for this important class of anticancer agents, since most proteins that cause cancer or are associated with the disease are buried inside cancer cells.
An Important Cancer Target
“This is a new approach for attacking an important cancer target with an antibody therapy. This is something that was previously not possible,” says David A. Scheinberg, Chair of the Sloan Kettering Institute’s Molecular Pharmacology and Chemistry Program and an inventor of the antibody. “Our research shows that you can use a monoclonal antibody to recognize a cancer-associated protein inside a cell, and it will destroy the cancer cell.”
ESK1 targets a protein called WT1, which is overexpressed in a range of leukemias and other cancers including myeloma, mesothelioma, breast cancer, ovarian cancer, and colorectal cancer.
WT1 is a critically important target for cancer drugs because it is an oncogenic protein, meaning that it supports the formation of cancer. In addition, it is found in very few healthy cells, so there are less likely to be side effects from drugs that target it.
Mimicking the Immune System
ESK1 was engineered to mimic the function of T cells, white blood cells that are key components of the immune system. T cells have a receptor system that is designed to recognize proteins that are inside the cell.
As proteins inside the cell get broken down as part of regular cellular processes, molecules known as HLA molecules carry fragments of those proteins — known as peptides — to the surface. When T cells recognize certain peptides on the surface of cells as abnormal, the T cells kill the diseased cells.
In the current study, the investigators showed that ESK1 was able to recognize the WT1 peptide even though the antibody itself did not enter the cells. ESK1 killed cancer cells in a test tube and also in mouse models for two different types of human leukemia.
“We were surprised that the antibody worked so well on its own,” says Dr. Scheinberg, senior author of the paper. “We had originally expected that we might need to use the antibody as a carrier to deliver a drug or a radioactive therapy to kill the cancer cells, but this was not necessary.”
Advancing to Clinical Studies
Additional studies must be done in the laboratory before ESK1 is ready to be used in clinical trials for patients. The monoclonal antibody was engineered to be completely human, which should shorten the time it takes to move the drug into the clinic. Researchers expect that the first clinical trials, for leukemia, could begin in about a year.
The antibody was developed under a collaborative effort between Memorial Sloan Kettering and Eureka Therapeutics, which have jointly filed for patent protection.
Research is also under way to target WT1 with vaccines and engineered T cells. These therapeutic approaches are currently in clinical trials at Memorial Sloan Kettering for leukemia, multiple myeloma, ovarian cancer, and mesothelioma.



