Our lab focuses on the generation of tissue structure through the collective action of cell populations.
During development, many features of tissue and body form are shaped by the reorganization of cell populations, where by individual cells coordinate their movements to produce a global change in tissue morphology. This tissue remodeling relies on the integration of multiple cellular processes, including cell polarity, motility, and adhesion. Moreover, these processes are directly modulated by communication between cells so that all members of a population move in the same way. The organized action of cell populations is fundamental to establishing body form and generating the distinct shapes and functions of different organs. These processes can also be coopted to promote the renegade cell movements that lead to tumor cell metastasis.
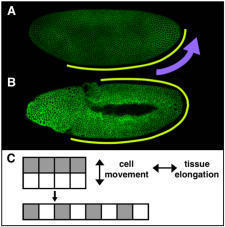
Elongating the Embryo: Convergent Extension in Drosophila
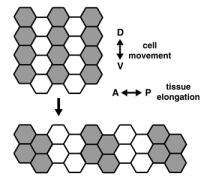
A dramatic example of coordinated cell movement is convergent extension, which drives elongation of the body axis in both vertebrates and invertebrates. During convergent extension in Drosophila — also known as germband extension (or gbex) — polarized cell movements cause the embryo to narrow along the D-V (back-to-front) axis and more than double in length along the A-P (head-to-tail) axis (Figure 1). These movements can be directly observed in living embryos. The key to this massive change in tissue structure is the polarized migration of individual cells, which move along the D-V axis, causing the germband to elongate perpendicular to the direction of cell movement (Figure 3).
We are using molecular, genetic, and cell biological approaches to understand the machinery that directs morphogenetic events. Our analysis is motivated by three central questions: How do cells establish asymmetry? How does this asymmetry mobilize the cytoskeleton to promote cell movement? And what is the spatial information that coordinates these movements across a multicellular population? An understanding of the cell rearrangements that occur during normal embryonic development will uncover general principles that build tissues and organs and can provide insight into how deranged versions of these processes contribute to human disease.
Dictatorship or Democracy?
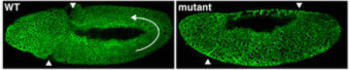
How do cells of the germband reach a collective decision about where to go? One possibility is that germband cells respond to an external directive from a distant tissue, such as a midline attractant that induces cells to move ventrally, or a pull from the posterior end that stretches the germband passively. These possibilities are unlikely, however, since the germband can still elongate in mutant embryos that lack these outside tissues (Irvine and Wieschaus, 1994).
While external forces are dispensable for cell rearrangement, patterning information within the germband is essential. For example, Even-skipped (Eve) and Runt are transcription factors expressed in stripes along the A-P axis and this striped expression is required for intercalation. If either gene is absent or expressed uniformly, germband extension comes to a halt. Moreover, if Eve stripes are artificially restricted to one part of the germband, cells in this region will intercalate independently of the rest of the tissue (Irvine and Wieschaus, 1994). Therefore, grass roots organization within the germband is sufficient to bring about local changes in cell position and, ultimately, a global change in tissue morphology.
Knowing Your Neighbors: How Do A-P Differences Drive D-V Cell Movement?
Eve and Runt are clearly required for cell rearrangement during germband extension, but these results present a paradox: These genes are present in A-P stripes, but germband cells move dorsoventrally — perpendicular to this spatial information. One potential explanation is that Eve and Runt stripes may act to establish key differences between neighboring cells along the A-P axis. These differences would then set in motion a massive cell sorting event, where by cells try to maximize contact with cells of the same type and minimize contact with cells perceived as “different.” Cells of a common stripe would coalesce into a group, forcing the germband to elongate. We are now using microarray analysis to identify the transcriptional targets of Eve and Runt that contribute directly to these differences between cells. Functional analysis of such target genes will provide information about how populations of cells work together to produce a global change in tissue structure.
Generating Asymmetry: Localized Proteins Polarize the Cell
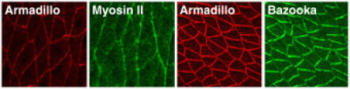
Oriented cell movements during convergent extension ultimately derive from the polarized localization of proteins that influence cell motility. A molecular description of this polarity is just beginning to emerge. We have shown that germband extension in Drosophila relies on a conserved set of asymmetrically distributed signaling and cytoskeletal molecules (Figure 5). The actin motor protein nonmuscle myosin II is enriched at vertical borders between neighboring cells along the A-P axis, which may prevent cells from moving in the wrong direction. Conversely, the Bazooka/PAR-3 protein is targeted to the horizontal D-V interfaces, consistent with a role in promoting cell motility. The mechanism by which these polarized proteins direct cell migration is not understood, but we imagine that localized proteins, such as Bazooka and myosin II, ultimately lead to differential regulation of the cytoskeletal structures and adhesive contacts that drive cell movement.
The Molecular Machinery At Work: Screen for New Components Involved in Cell Rearrangement
While few molecules that mediate morphogenesis are now known, rapid genetic approaches available in Drosophila provide an opportunity to uncover essential components in this process, in the absence of any information about their identity. Since germband extension is one of the earliest events in Drosophila embryonic development, we are able to use the relatively blunt approach of removing large portions of the genome and asking whether gbex is disrupted by knocking out 50 to 100 genes at once. By systematically analyzing a set of overlapping deletions, we discovered ten loci that are required for cell rearrangement and are now cloning the relevant genes (Figure 6). We are particularly interested in genes that generate polarity; genes that translate this polarity into cell movement; and genes that coordinate these movements across a multicellular population.
Gbex, Flies, and Videotape: Live Imaging of Drosophila Embryos
To understand the dynamic processes that organize cell populations in vivo, we are using time-lapse confocal microscopy to follow cell behavior in living embryos. This visualization of cell morphology during tissue remodelling can provide insight into basic unanswered questions: Do cells move by actively crawling either on a substrate or over each other, and do protrusive structures, such as filopodia and lamellipodia, generate traction in this process? Alternatively, do cells use a form of selective adhesion to cling to preferred neighbors and break free of less desirable ones? By simultaneously following the localization of proteins tagged with different variants of GFP, we can reconstruct the precise molecular steps that underlie the cellular events of morphogenesis. Moreover, we can use mutant embryos to examine how these events are altered in the absence of specific proteins. Ultimately, we hope to reconstruct a chain of events that will allow us to predict the behavior of a cell based on the machinations of its component proteins.