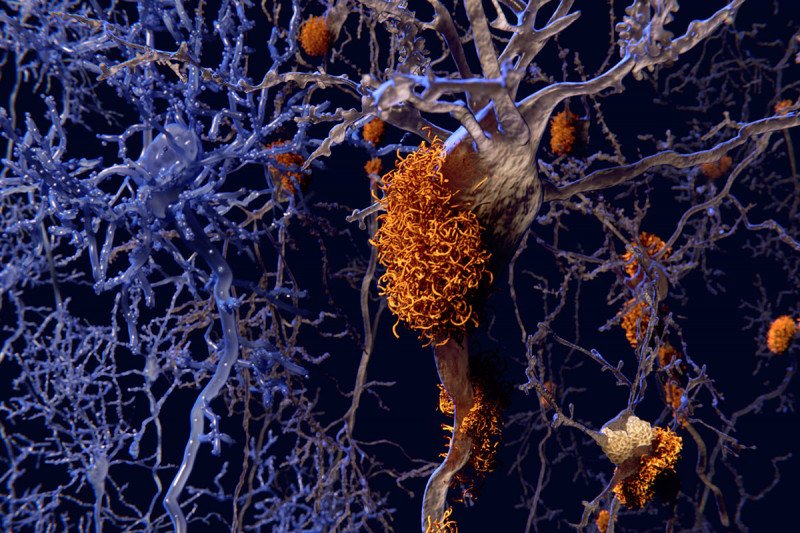
Alzheimer’s disease is a neurodegenerative condition that is characterized by the buildup of clumps of beta-amyloid protein in the brain. Exactly what causes these clumps, known as plaques, and what role they play in disease progression is an active area of research important for developing prevention and treatment strategies.
Recent studies have found that beta-amyloid has antiviral and antimicrobial properties, suggesting a possible link between the immune response against infections and the development of Alzheimer’s disease.
Chemical biologists at the Sloan Kettering Institute have now discovered clear evidence of this link: A protein called IFITM3 that is involved in the immune response to pathogens also plays a key role in the accumulation of beta-amyloid in plaques.
“We’ve known that the immune system plays a role in Alzheimer’s disease — for example, it helps to clean up beta-amyloid plaques in the brain,” says Yue-Ming Li, a chemical biologist at SKI. “But this is the first direct evidence that immune response contributes to the production of beta-amyloid plaques — the defining feature of Alzheimer’s disease.”
In a paper published September 2 in Nature, Dr. Li and his team show that IFITM3 alters the activity of an enzyme called gamma-secretase, which chops up precursor proteins into the fragments of beta-amyloid that make up plaques.
They found that removing IFITM3 decreased the activity of the gamma-secretase enzyme and, as a result, reduced that number of amyloid plaques that formed in a mouse model of the disease.
Mounting Evidence for a New Hypothesis
Neuroinflammation (inflammation in the brain) has emerged as an important line of inquiry in Alzheimer’s disease research. Markers of inflammation, such as certain immune molecules called cytokines, are boosted in Alzheimer’s disease mouse models and in the brains of people with Alzheimer’s disease. Dr. Li’s study is the first to provide a direct link between this inflammation and plaque development — by way of IFITM3.
Scientists know that the production of IFITM3 starts in response to activation of the immune system by invading viruses and bacteria. These observations, combined with the new findings from Dr. Li’s lab that IFITM3 directly contributes to plaque formation, suggest that viral and bacterial infections could increase the risk of Alzheimer’s disease development. Indeed, Dr. Li and his colleagues found that the level of IFITM3 in human brain samples correlated with levels of certain viral infections as well as with gamma-secretase activity and beta-amyloid production.
Age is the number one risk factor for Alzheimer’s, and the levels of both inflammatory markers and IFITM3 increased with advancing age in mice, the researchers found.
They also discovered that IFITM3 is increased in a subset of late onset Alzheimer’s patients, meaning that IFITM3 could potentially be used as a biomarker to identify a subset of patients who might benefit from therapies targeted against IFITM3.
The researchers next plan is to investigate how IFITM3 interacts with gamma-secretase at the molecular and atomic levels and how it is involved in neuroinflammation in animal models. They will also explore IFITM3 as a biomarker for the disease and as a potential target for new drugs designed to treat it.