The most common type of breast cancer is called estrogen receptor (ER) positive. It accounts for about 70% of breast cancer diagnoses. Women with this form of the disease are usually treated with drugs that prevent estrogen from fueling the cancer’s growth. However, when the cancer spreads, it usually becomes resistant to these hormonal therapies.
In recent years, treatment of ER-positive breast cancer took a step forward with CDK4/6 inhibitors. These drugs stop the growth of breast cancer cells by targeting enzymes that are important in cell division. When given in combination with hormone therapy, CDK4/6 inhibitors have shown remarkable effectiveness in treating people with ER-positive breast cancer. In 2017 alone, the US Food and Drug Administration approved three CDK4/6 inhibitors.
But in 10 to 15% of women, CDK4/6 inhibitors don’t work at all. Even people who initially respond to the drugs often develop resistance.
Now a study led by Memorial Sloan Kettering physician-scientist Sarat Chandarlapaty has discovered the main reason for resistance to CDK4/6 inhibitors. By analyzing breast cancer samples and doing tests in laboratory models, the investigators identified two genes that play a critical role in promoting this resistance: FAT1 and RB1. Mutations in either of these genes can lead to resistance in two different ways.
The researchers published this discovery today in Cancer Cell.
“This points us to the next steps in developing a new generation of therapies to overcome resistance,” Dr. Chandarlapaty says. “It also helps us identify breast cancers that may need other approaches besides CDK4/6 inhibitors.”
Narrowing the Resistance Focus
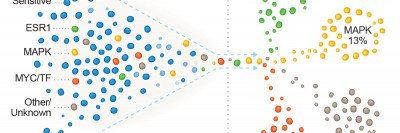
In September 2018, Cancer Cell published a catalog of genetic changes that MSK researchers had associated with hormone resistance in metastatic ER-positive breast cancer. (Metastatic cancer has spread to a different location in the body from where it started.) They found these changes through genetic sequencing of tumors using MSK-IMPACT™. This test looks for mutations in 468 genes associated with cancer. It is the largest clinical genetic study of hormone resistance in people with metastatic ER-positive breast cancer to date.
In the new study, the researchers looked specifically at resistance after treatment with CDK4/6 inhibitors.
“We knew that in 20 to 30% of people, breast cancer progresses early after treatment with these drugs, but we didn’t know what genes might be involved,” says physician-scientist Pedram Razavi, an author on both the September paper and the new study. “We did the genetic analysis of ER-positive breast tumors and correlated the data with how well the women fared under CDK4/6 treatment.”
FAT1 and RB1 are both tumor suppressors. (They prevent cancer from developing.) The team found that in women treated with CDK4 inhibitors, those whose tumors had mutations in FAT1 or RB1 were likely to have their cancer grow or spread sooner. This suggested the mutations gave rise to resistance to the drugs. Earlier studies had associated loss of RB1 function with resistance, but the FAT1 connection was a surprise.
Studying the tumor samples revealed that loss of FAT1 boosts production of the CDK6 enzyme. The increased CDK6 levels appeared to cause the resistance. The researchers confirmed this idea using human breast tumor samples grafted into mice. When they blocked CDK6 production, the tumors became sensitive to CDK4/6 inhibitors.
The researchers are now trying to develop drugs that will counteract the rise in CDK6 levels due to FAT1 and RB1 mutations.
In the shorter term, doctors can begin by identifying people with these mutations, since they should probably not receive CDK4/6 inhibitors. These women would likely resist the drugs from the start of treatment. Dr. Chandarlapaty stressed that further studies are needed to validate this finding first.
The Power of Collaboration in Research
“This finding really shows the value of genomic studies matched with clinical data,” Dr. Chandarlapaty says. “With only genetic information, we would not have known where to look for the mutations causing the resistance. Incorporating the clinical outcomes showed that the effect of these two mutations is striking.”
Dr. Razavi says that the study also illustrates the advantages of collaboration. At MSK, researchers were able to look at patient samples and treatment data, find genetic associations, and then confirm them in the laboratory.
“It’s very common to find associations in clinical data, but you don’t know what they mean or how a mutation is promoting the cancer,” he says. “You have to be able to prove it with controlled experiments. We were able to do that in a few weeks.”
“I think this is a really robust example of how we can learn from our patients and go back and forth between the bedside and the lab,” Dr. Chandarlapaty adds. “I’m very proud of how MSK enables us to do that.”