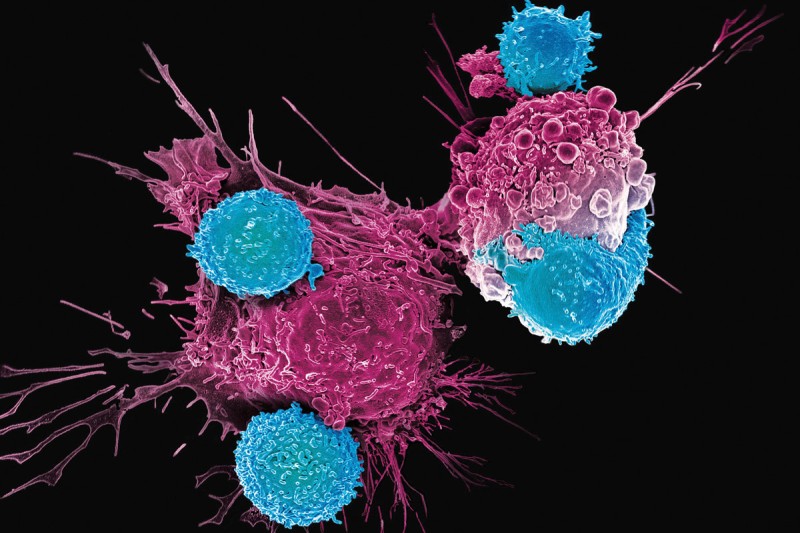
This electron microscopic image shows CAR T cells (blue) attacking cancer cells (pink). Courtesy of Dr. Prasad Adusumilli.
Giving one dose of a treatment that will stay in a patient’s body to help it continue to fight cancer may seem like mission impossible. But it’s not. This type of immunotherapy, called CAR T cell therapy, is one of the most exciting fields in cancer treatment today.
What Are CAR T Cells and How Do They Treat Cancer?
Think of it as creating “cellular assassins,” trained to eliminate cancer cells. T cells from a patient’s own immune system are removed and then outfitted in the lab with special tools that recognize specific targets on the surface of a cancer cell. When these armed cells are put back into the patient, they patrol the bloodstream like elite soldiers on search-and-destroy missions attacking those targets, leaving minimal collateral damage, and resuming the action if the cancer cells return.
MSK’s CAR T Cell Discoveries and Further Research
For more than two decades, scientists at Memorial Sloan Kettering Cancer Center (MSK) have been pioneers in this field, designing and conducting clinical trials using CAR T cell therapy. They’ve invented as many as 20 CAR T technologies so far. MSK discoveries have made CAR T cell therapy especially effective in treating patients with blood cancers, including certain leukemias and lymphomas, as well as multiple myeloma.
But there are major hurdles currently keeping CAR T cell therapy from helping more patients:
- The cells can take weeks to prepare and are expensive because they are engineered individually for each patient.
- Many people don’t respond to the treatment, or the cancer returns after it works. It also can have serious side effects in the short term.
- CAR T cells haven’t worked well against solid tumors, which make up the vast majority of cancers.
Every day, across MSK, scientists and clinicians are working to overcome the obstacles for this treatment, whose full name is “chimeric antigen receptor T cell therapy.” The “antigen” is a protein that exists in abundance on cancer cells but not normal cells. CAR T cells are engineered to selectively target these antigens, destroying the cancer.

Dr. Michel Sadelain recently received the prestigious Breakthrough Prize in Life Sciences for his pioneering work to develop CAR T cell therapy.
“By investigating how T cells are wired and what receptors make them work, our laboratories can keep pushing the limits of this revolutionary treatment,” says physician-scientist Michel Sadelain, MD, PhD, who first engineered T cells 30 years ago. As Director of the Center for Cell Engineering and through his lab at the Sloan Kettering Institute, Dr. Sadelain has continued leading the field.
His lab is one of more than a dozen throughout MSK focused on improving CAR T cell therapy in the following ways:
Preventing T Cell Exhaustion: MSK Helps Them Keep Up the Fight
The T cells can lose their potency over time — a phenomenon known as T cell exhaustion — but Dr. Sadelain’s lab found a way to keep up the fight by inserting a molecule called 1XX into the T cell genome at a precise location called TRAC. His team used the new gene-editing technology CRISPR for this groundbreaking feat of engineering,
The laboratory advance is now coming to fruition in patients. In early 2023, MSK launched a clinical trial, led by hematologic oncologist Jae Park, MD, to test these fortified CAR T cells against a type of lymphoma. The translation from lab to clinic was made possible by Isabelle Rivière, PhD, and the Cell Therapy and Cell Engineering Facility at MSK. “If the cells are more potent and last longer, we ultimately would need fewer of them, which could make manufacturing easier and less expensive,” Dr. Park says.
How MSK Is Overcoming CAR T Cell Resistance
Another major challenge for CAR T is that cancers develop resistance to the treatment by making less of the target antigens. This problem, known as antigen escape, enables the cancer to come roaring back. But Dr. Sadelain and colleagues used CRISPR to boost the sensitivity of the T cells to antigens by at least tenfold, enabling the CAR T cellular assassins to finish the job, even with fewer targets guiding their way.
These redesigned cells are called HLA-independent T cell receptor (HIT) T cells.
“This could really expand the field,” Dr. Sadelain says. “We may no longer be limited to targeting antigens that are abundant for CAR T cells to work.”
Allowing CAR T Cells To Penetrate Solid Tumors
A major reason CAR T cell therapy hasn’t worked on solid tumors is that they are often difficult for T cells to penetrate. Like a moat around a fortress, tumors are also surrounded by cells and molecules that hinder the T cells until they run out of steam.
But the laboratory of physician-scientist Prasad Adusumilli, MD, is trying to solve this problem for CAR T cells in multiple ways, including:
- Delivering CAR T cells directly into the chest cavity for tumors caused by mesothelioma, a rare cancer in the lining surrounding organs.
- Using low-dose radiation to help CAR T cells penetrate tumors.
- Genetically engineering the CAR T cells to contain a “decoy receptor” that foils cancer cells from blocking the immune cells until they become exhausted.
- Co-opting one of cancer’s own weapons by adding a mutation in a gene called c-KIT to the CAR T cells. When given to animals with human tumors from mesothelioma, lung, and prostate cancers, these new tricked-out cells appeared to be more effective than conventional T cells.
Another approach to make CAR T cells work for solid tumors is by turning the cell into a “micropharmacy.” An entirely new type of CAR T cell has been designed by physician-scientist David Scheinberg, MD, PhD, Chair of the Center for Experimental Therapeutics, in collaboration with chemical biologist Derek Tan, PhD, Chair of the Chemical Biology Program.
These T cells can activate a toxic drug payload directly at a tumor, killing both tumor cells that contain the cancer marker as well as those cancer cells nearby that do not have the marker. Fittingly, the name for these cells is SEAKER (Synthetic Enzyme-Armed KillER).
“These cells combine the target-seeking power of immune cells with the ability to generate a potent anti-cancer drug right at the site of a tumor. It’s a one-two punch on the cancer,” Dr. Scheinberg says.
Peering Inside Cancer Cells

Dr. Christopher Klebanoff is investigating ways to enable T cells to detect mutated proteins inside cancer cells.
Currently, CAR T therapies can only engage target proteins located on the outside of cancer cells, a feature that limits their effectiveness and safety, especially against solid tumors. MSK physician-scientist Christopher Klebanoff, MD, is focused on an innovative, next-level approach.
His team is researching ways to genetically reprogram T white blood cells to recognize mutated cancer-causing proteins located on the inside of cancer cells — using a technique called T cell receptor therapy. This approach engineers T cells to enable them to survey the interior of cells in search of rogue proteins that are unique to tumors.
“We’re using a genetic engineering approach to help supercharge T cells in a new way,” says Dr. Klebanoff. “It’s akin to giving immune cells X-ray vision.”
Making CAR T Cell Therapy Easier for Patients — and More Accessible

Dr. David Scheinberg is collaborating with other MSK researchers to retool CAR T cells so they can be more potent and less toxic.
The need for CAR T cells to be custom-made for each patient makes the treatment expensive and time-consuming. That’s why researchers are striving to make standardized CAR T cells. An “off-the-shelf” therapy would use cells taken from healthy donors — known as an allogeneic transplant — which then would be genetically modified and stored in large batches, ready for treatment.
“CAR Ts may never be widely used if they have to be made one at a time, per patient,” Dr. Scheinberg says. “We really want something that’s waiting in a freezer — you order it, it’s thawed, and you infuse it into the person the next morning.”
Multiple myeloma specialist Sham Mailankody, MBBS, has reported encouraging results using donor CAR T cells to treat multiple myeloma in a small group of patients. These cells were modified using a virus.
Reducing the Risk of Graft-Versus-Host Disease With Donated CAR T Cells
In the lab, a more precise engineering technique may be able to prevent the downside of using donor CAR T cells — a serious side effect called graft-versus-host disease, which occurs when donor cells attack the patient’s normal cells. By using CRISPR technology, MSK researchers are studying how to tweak the CAR T cells to disable the part that triggers this side effect.
In turn, to keep a patient’s immune cells from attacking donor CAR T cells, Dr. Scheinberg’s lab has developed new cells that could fend off this assault, using what he calls a “shield CAR T cell.”
“These CAR Ts are designed specifically to be off-the-shelf because that is where rejection is such an important issue,” says Dr. Scheinberg.
No doubt, making CAR T cell therapy a truly transformative cancer treatment requires the continued drive and ingenuity of MSK scientists. But the pace of discovery is picking up, thanks to new technologies like CRISPR. Scientists who once only imagined changing the genes of a cell are now able to do so. With exquisite precision, they can arm immune cells to continually attack cancer. It’s a mission that’s now possible.
Dr. Sadelain holds the Stephen and Barbara Friedman Chair.
Dr. Scheinberg holds the Vincent Astor Chair.
Dr. Tan holds the Eugene W. Kettering Chair.