Physician-scientist Prasad Adusumilli specializes in treating tumors in the chest.
The immunotherapy treatment known as CAR T cell therapy, while still experimental, is showing promise for treating blood cancers such as leukemia. But so far, its effectiveness against solid tumors has been limited.
Now research from Memorial Sloan Kettering investigators done in mice has suggested an approach that appears to provide a more durable, long-lasting result for solid tumors: combining CAR T cell therapy with another immunotherapy treatment called checkpoint blockade.
“MSK is leading the world in developing both of these types of immunotherapy,” says MSK physician-scientist Prasad Adusumilli. “By combining CAR T cells with checkpoint blockade, we have demonstrated a new treatment strategy that can greatly benefit patient care.” Dr. Adusumilli is the senior author of a new study published July 25 in the Journal of Clinical Investigation showing these results.
Treating Patients with a “Living Therapy”
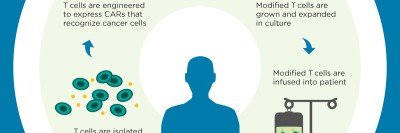
The CAR T cell technique involves filtering white blood cells called T cells from a patient’s blood and introducing a new gene into those cells. The gene enables the T cells to recognize a specific protein that’s present on cancer cells. The CAR T cells (CAR stands for chimeric antigen receptor) are then grown in the laboratory and infused back into the patient, after which they seek out and destroy the cancer. Dubbed a “living therapy,” this approach was first developed by MSK physician-scientist Michel Sadelain, who is a coauthor on the new paper.
The CAR T cell trials for leukemia at MSK target a protein found on leukemia cells called CD-19. Later work from Drs. Adusumilli and Sadelain demonstrated that T cells engineered to target a protein called mesothelin could be effective in treating solid tumors. Additionally, their research showed that these CAR T cells were more effective if they were infused directly into the chest cavity in patients with cancers in the chest. Based on that work, MSK launched a clinical trial for patients with lung cancer, breast cancer, and mesothelioma that have the mesothelin protein expressed on the tumor.
To mimic a clinically relevant scenario, researchers designed experiments by purposefully administering a very low dose of CAR T cells. “In the beginning the CAR T cells effectively kill the tumor,” Dr. Adusumilli explains. “But after a period of time, although they are persistent within the tumor, these cells are functionally exhausted.” The reason is that the cancer cells under attack produce a molecule called PD-L1 that engages a “brake” on the T cells and shuts them off.
Developing a Combination Immunotherapy Approach
During the same years that Dr. Sadelain and his colleagues were developing CAR T cell therapy, other investigators at MSK, including melanoma expert Jedd Wolchok, were developing another immunotherapy known as checkpoint blockade. The idea behind this approach is that by finding a way to take the brakes off the body’s immune cells, their innate ability to recognize and attack cancer cells could be greatly enhanced. There are now several checkpoint blockade drugs approved for use in cancers including melanoma, lung cancer, kidney cancer, and bladder cancer, as well as lymphoma.
Led by Dr. Adusumilli, the CAR T cell team had the idea to combine checkpoint blockade with their modified T cells to test whether the combination could lead to longer-lasting benefits.
In the new JCI study, the researchers showed in mouse models that this approach was indeed more effective. Adding a checkpoint drug to the treatment blocked the interaction between PD-L1 on cancer cells and the PD-1 receptor on the engineered T cells, thus preventing the cancer from shutting the T cells off and leading to a longer-lasting effect.
In the current trial that is already ongoing for chest tumors, Dr. Adusumilli plans to add a checkpoint blockade drug to the regimen. Another trial expected to launch soon for breast cancers that express mesothelin may also use this approach.
Next-Generation CAR T Cells
Dr. Adusumilli’s team decided to take their work one step further and engineer the T cells so that the checkpoint blockade was built in — creating what they call stress-resistant CAR T cells.
“We did this in a clever way by putting in a PD-1 decoy receptor, which allowed the T cells to resist the signal from PD-L1 and continue functioning in the mice,” he says. Going forward, he is planning new clinical research studies in which the CAR T cells would be engineered to contain the decoy receptor, “so that patients would only need to be subjected to a single therapy,” he adds.
“The knowledge that MSK has gained from conducting CAR T cell trials for blood cancers led by the Cellular Therapeutics Center has helped me immensely in my research,” he adds. “At the same time, continuing work by Dr. Wolchok and his group is paving the way to better understand checkpoint blockade. Working at MSK gives us the unique opportunity to learn from each other in real time, and this quickly benefits patient care.”