Our lab develops new minimally invasive image-guided cancer treatments:
- Methods for improving the anti-tumor immune response after embolization or ablation.
- Genetic, imaging, and other tests to predict response after embolization or ablation.
- New devices for endoluminal ablation (ureter, bile ducts, bronchi, bowel).
- New intra-arterial therapies for liver and pancreatic cancer.
- Transgenic pig model for liver and pancreatic cancer.
- Imaging technologies for rapid diagnosis from biopsy specimens.
- Outcomes after cancer interventions.
- Evidence-based cancer imaging.
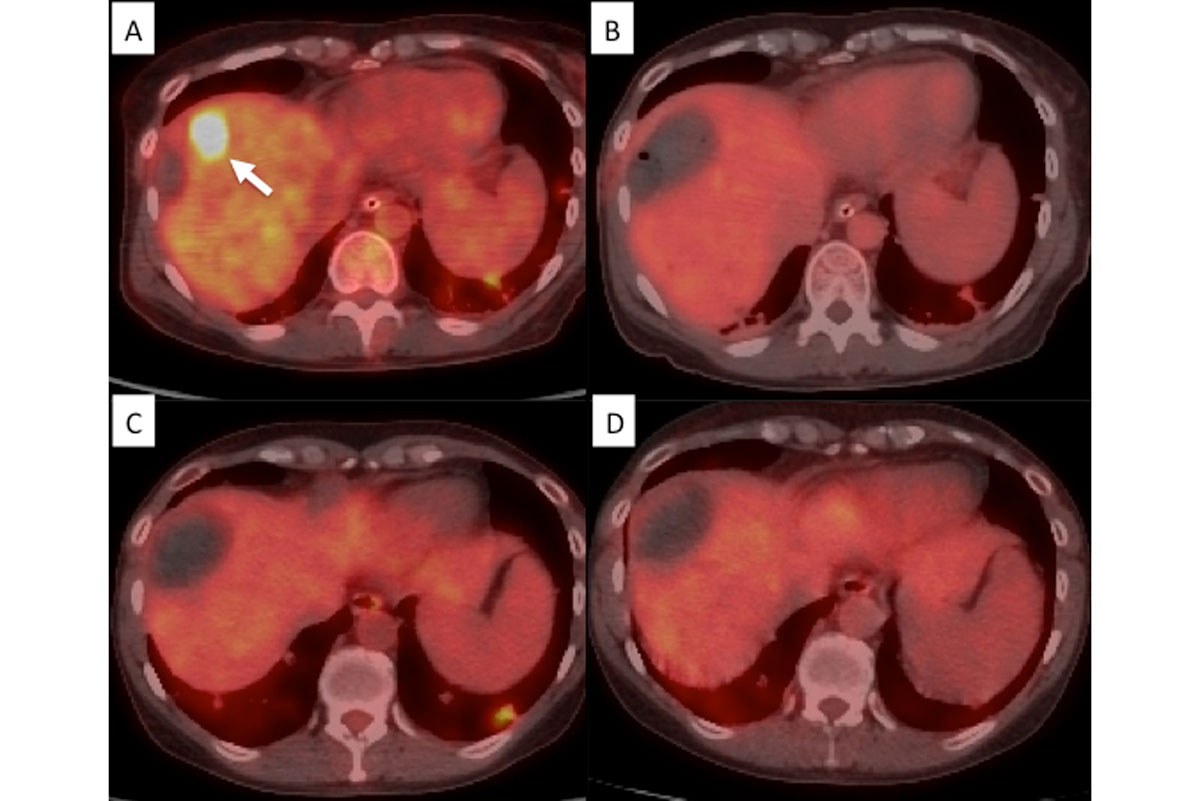
PET/CT guided ablation in a woman with a liver metastasis from colon adenocarcinoma treated with radiofrequency ablation. A-PET/CT shows a liver metastasis. B-Evaluation on procedure table immediately after ablation shows no residual uptake. C- Subsequent PET/CT at 6 months illustrating no uptake concordant with previous findings (true negative). D- PET/CT at 12 months shows no remaining.
Stephen Solomon’s research is in developing and improving minimally invasive, image-guided treatments for cancer. He incorporates molecular imaging into interventional therapy with techniques that determine viability at the time of treatment. The use of interventional PET/CT has allowed retreatment while patients are still undergoing a procedure to ensure completeness of treatment when the patient leaves the procedure suite. He also uses image-guided techniques to identify early tumors in genetically at-risk patients before tumors spread.
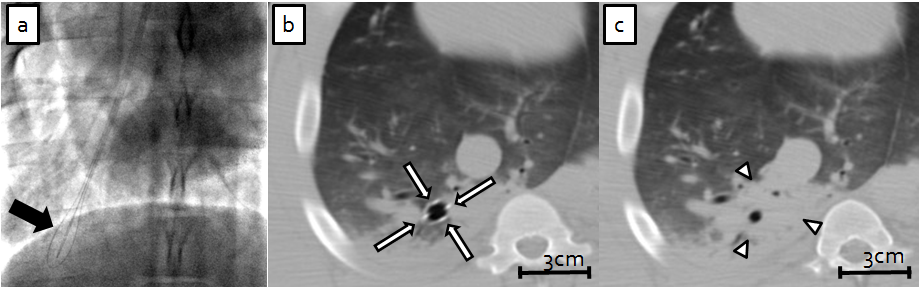
Endobronchial ablation.
Dr. Solomon studies the effects of electric pulses on malignant and healthy tissue, and its interaction with wound healing and inflammation. He uses these findings to develop novel ablation therapies and drug delivery techniques for the treatment of tumors in the hollow organs of the genitourinary, gastrointestinal and respiratory tracts.
Constantinos Sofocleous develops tissue biomarkers and corresponding image biomarkers that can be used as predictors of outcomes after hepatic tumor ablation. He has shown that identification of viable or prolific tumor cells on a post-ablation biopsy specimen is a strong independent predictor of local tumor recurrence. His efforts are focused on the development of intraprocedural methods of treatment assessment using tissue and imaging biomarkers in an effort to improve local cancer therapies. He is in particular focused on the treatment of colorectal cancer tumors in selected patients with oligometastatic disease that can be treated with image-guided therapies ranging from ablation to intraarterial therapies. His efforts extend to the development and application of a colon cancer specific nomogram incorporating clinical and molecular characteristics that influence the expression of biomarkers and target tumor specific response to locoregional therapies.
Joseph Erinjeri’s research focusses on understanding and targeting the immune responses to image-guided intervention. The objective of this work is to harness the immune responses to image-guided ablation and embolization of tumors to produce more effective clinical treatments that not only have local effects on tumors, but systemic effects as well. The immunobiology of embolization and thermal ablation is not well understood, although these techniques are the mainstay of therapy in interventional radiology for the local control of unresectable and/or progressive malignancy. As principal investigator of several extramurally funded grants, Dr Erinjeri identified interleukin (IL)-6 as a key cytokine that underlies the inflammatory response following thermal ablation. He also measured T-cell repertoires following ablation and embolization, setting the stage to study combination immunotherapy / interventional procedures to improve T-cell function during embolization and ablation. By understanding the basic immune responses to image guided ablation and embolization, Dr Erinjeri will have the ability to develop rational adjuvant therapies for interventional procedures that increase efficacy and decrease recurrence.
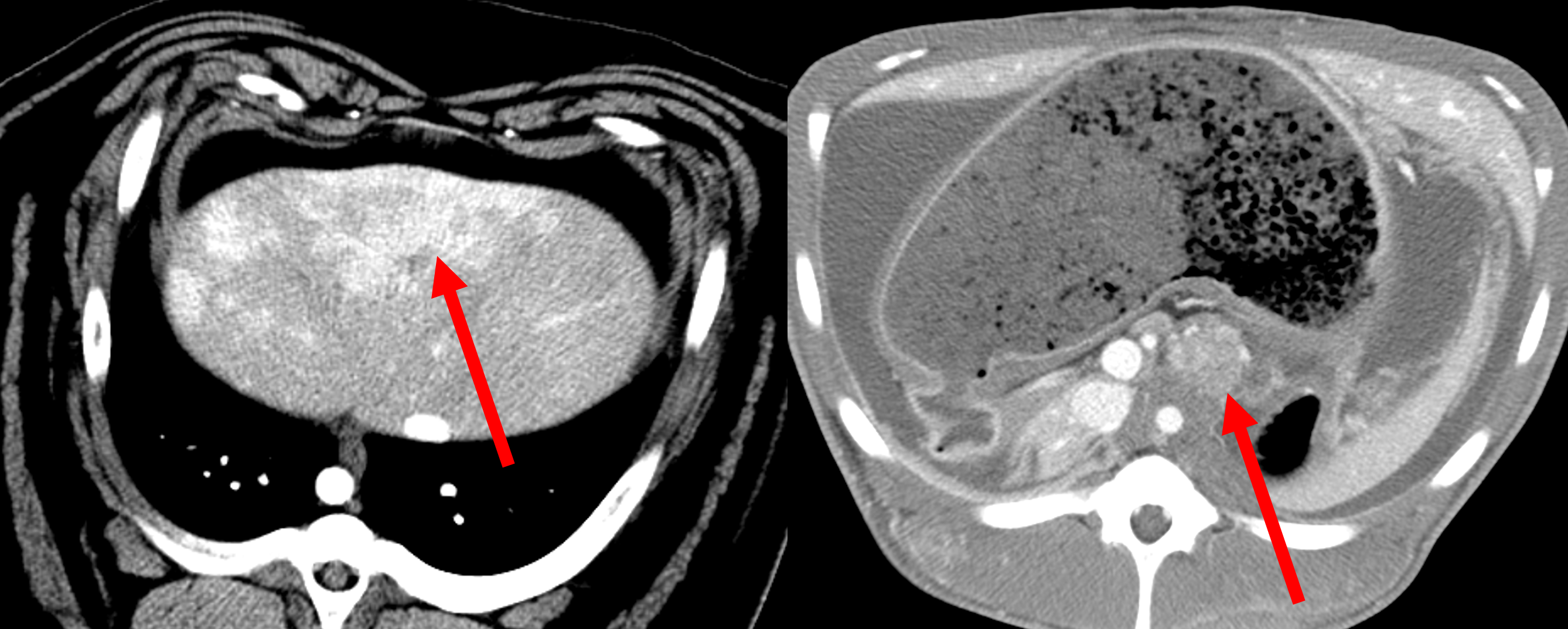
Pig tumor models. Liver (left) and pancreatic (right) tumors in an Oncopig, a transgenic pig with locally inducible Kras and p53 mutations.
Hooman Yarmohammadi is developing new intra-arterial treatments for pancreatic cancer, which he is testing in a pig tumor model. He has also found a drug that is able to undo the changes that occur in liver cancers that make them resistant to embolization therapy. Essentially, this treatment “chokes” the tumors by depriving them of oxygen-rich blood. But, incredibly, liver cancer cells change and learn how to live and reproduce without oxygen—a phenomenon called the “Warburg Effect” that makes embolization less effective. The drug Dr. Yarmohammadi has identified blocks the Warburg Effect, and he is now evaluating its safety and effectiveness. Preliminary data from these studies are encouraging, and he hopes to move the drug forward into clinical trials to assess its value in patients.
Etay Ziv focuses on understanding mechanisms of resistance and sensitivity to local-directed therapies in order to develop adjuvants to improve patient outcomes. On the clinical side, he is identifying signaling pathways that predict response to embolization, radioembolization, and thermal ablation. In the lab, he is using cell lines and patient derived tumor models to dissect these pathways. Dr Ziv is also developing tools to interrogate the spatial distribution of tumor heterogeneity. Finally, he is developing computational radiomic tools, including a project to track lung nodules to predict patient outcome and a project to track changes in the human brain connectome after chemotherapy and radiation.
Amy Deipolyi works on developing new effective treatments for breast cancer, with a specific focus on treating metastatic disease in the liver. One treatment for breast cancer liver tumors is radioembolization, a procedure involving the delivery of radioactive particles directly into the liver lesions. It is not known which patients will benefit most from this treatment. Dr. Deipolyi studies biomarkers predicting response, and ways to make this treatment more effective for liver lesions and for tumors outside the liver.