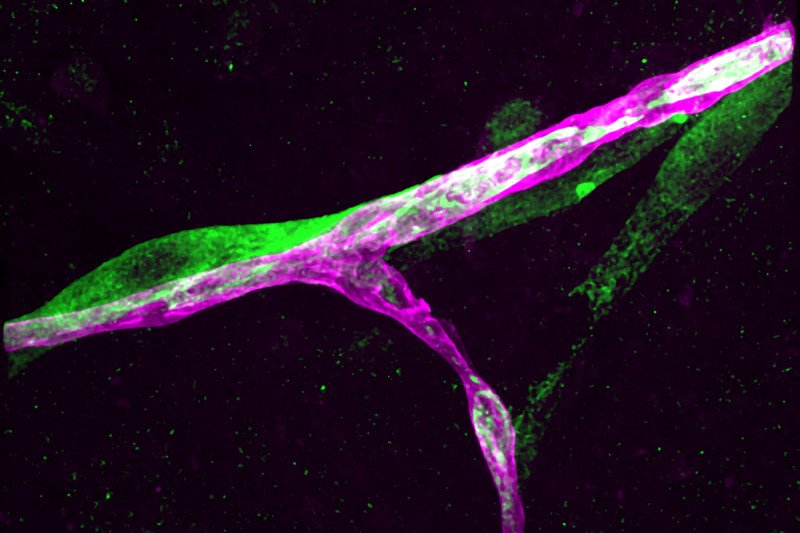
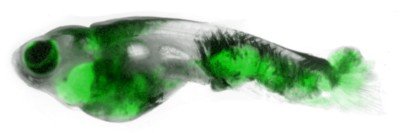
The Alan and Sandra Gerry Metastasis and Tumor Ecosystems Center (GMTEC) brings together a diverse group of Memorial Sloan Kettering’s basic scientists and clinicians who are conducting research with a focus on metastatic traits, brain metastasis, tumor ecosystems, the metastatic microenvironment, and latent metastasis. GMTEC supports their research initiatives with funds that are raised and targeted specifically for metastasis research.
In this section, you will find information about GMTEC, its mission and scope, leadership and activities, the various funding opportunities offered through the center, and past projects funded. The center’s new Single-Cell Analytics Innovation Lab (SAIL) is also featured, with details on current collaborations, resources available, and support and collaboration opportunities for researchers.
Spotlight on Past Trainees
In keeping with GMTEC’s mission to stimulate and facilitate research efforts on the causes, mechanisms, and treatments of metastasis, GMTEC encourages former Fellows to continue this pursuit after their training at Sloan Kettering Institute has concluded. Our former Fellows are scattered around the globe pursuing both clinical and research careers. We are proud to highlight a few of them here:
Dr. Pereira was a GMTEC Fellow from 2019-2021. Her GMTEC fellowship was fundamental for studying the synergy between HER2-targeted therapies and membrane trafficking modulation in advanced metastatic gastric cancer. In June 2021, she joined Washington University School of Medicine as an Assistant Professor of the Mallinckrodt Institute of Radiology. Patrícia’s research program centers on using immunoimaging toolboxes, in concert with complementary genetic and proteomic approaches, to investigate how regulation of membrane receptors can function in metastasis and their microenvironment at the most basic level and at the level of drug resistance.

Dr. Chan was the GMTEC Gerry Fellow from 2019-2021. He is now an Instructor on the Thoracic Oncology Service at Memorial Sloan Kettering where he continues to leverage single-cell technologies to study lineage plasticity as a mechanism of metastasis and acquired resistance in lung and prostate cancer. The GMTEC fellowship was instrumental in profiling the single-cell heterogeneity of tumor phenotypes and the surrounding immune microenvironment in small cell lung cancer (SCLC), recently published in the Nov. 2021 issue of Cancer Cell. He now endeavors to use this de novo SCLC single-cell atlas as a comparative reference to study histological transformation from lung adenocarcinoma to SCLC. In parallel, he also studies a similar phenomenon of tumor plasticity in a murine organoid model of RB/TP53-mutant prostate adenocarcinoma. He found that this plasticity results in a mixed basal/luminal phenotype that is driven by FGFR and JAK/STAT signaling and resistant to androgen inhibition, with dual FGFR and JAK inhibition reversing plasticity and restoring androgen sensitivity. Studying this process in the context of the tumor microenvironment in genetically engineered mouse models and clinical samples demonstrates further diversification of EMT-like and neuroendocrine lineages from a stem-like luminal subpopulation (bioRxiv Nov. 2021 pre-prints available here). The GMTEC fellowship was critical to all these studies and Dr. Chan’s academic development.

Assistant Member, Molecular Pharmacology Program, Sloan Kettering Institute
The Ganesh lab develops sophisticated patient-derived organoid models of gastrointestinal cancer to understand the molecular mechanisms of cellular reprogramming during tumor regeneration in metastasis and after therapy. Dr. Ganesh’s research program integrates single cell and spatial profiling of patient samples with mechanistic interrogation in patient-derived organoid and mouse models, with the goal of developing novel therapeutic approaches for advanced cancer.