Zebrafish models of cancer


Casper
Using a zebrafish model of melanoma, we use state of the art genetic engineering and high-resolution imaging to dissect these interactions. Our goal is to understand the mechanisms that allow tumors to successfully disseminate and take hold in new locations, which is the major cause of cancer mortality.
The lab is interested in the ways in which heterogeneous cell populations interact to promote tumor progression and metastasis. These cell-cell interactions can be between tumor cells themselves, but also between tumor cells and the microenvironment. A major emphasis is the way in which these interactions can alter cell state through changes in transcriptional programs. We use transparent strains like casper to enhance our ability to image these dynamic cell-cell interactions in vivo.
From melanocytes to melanoma: embryonic neural crest programs and lineage addiction

mitf-BRAFV600E;p53-/-
Melanoma arises due to oncogenic transformation of melanocytes, which are the pigment producing cells in the skin. DNA mutations in oncogenes like BRAFV600E are a critical component of melanoma initiation, but an equally important part is the transcriptional landscape, or lineage, in which those mutations occur. To study this, we developed a zebrafish model of melanoma in which the BRAFV600E oncogene is expressed specifically in melanocytes. We discovered that nearly all melanomas express a suite of genes like crestin and SOX10 that are characteristic of the neural crest, which is the embryonic progenitor population that ultimately gives rise to the differentiated melanocytes (White, et al, Nature 2011 and Kaufman, et al, Science 2016). Genetic ablation of neural crest genes such as SOX10 strongly abrogates melanoma growth, confirming that this embryonic lineage program is an essential component of melanocyte transformation. We also discovered that chemicals which inhibit the enzyme DHODH can suppress this embryonic neural crest crest gene program, and offer a potential therapeutic avenue for targeting these embryonic programs in melanoma. The necessity of these embryonic programs in melanoma initiation is a potent example of lineage addiction, in which the tumor cells become dependent on the transcriptional programs present in the melanocyte lineage.
Ongoing work: We are continuing to explore the ways in which embryonic lineage programs play distinct roles in melanoma pathogenesis and metastasis. A major focus is discerning how the differentiation state of the cell (i.e. neural crest versus melanocyte) affects the capacity for BRAFV600E to be oncogenic.
Microenvironmental influences on melanoma progression
The lineage programs are strongly influenced by the local microenvironment, which is different in each anatomic site. Within the skin, melanoma cells are surrounded by keratinocytes which secrete molecules such as EDN3 that promote the ability of nascent melanomas to invade and metastasize. Using a CRISPR loss of function screen, we showed that deletion of EDN3 or its synthetic enzyme ECE2 from the microenvironment markedly suppresses tumor growth (Kim, et al, Nature Communications 2017). As the melanomas invade more deeply, they come into contact with subcutaneous tissues which are primarily composed of adipocytes. The nature of the melanoma-adipocyte interaction is largely unexplored, but we have shown that adipocytes can directly donate long chain fatty acids into melanoma cells via the FATP fatty acid transporter (Zhang, et al, Cancer Discovery 2018). Melanoma cells which overexpress FATP proteins have increased lipid content and metabolically reprogram the tumor into an invasive, aggressive state. Both genetic and pharmacologic inhibition of FATP proteins blocks the melanoma-adipocyte interaction, highlighting an important new mechanism for therapeutic targeting of the tumor microenvironment. In each of these distinct anatomic sites, the melanocytes and melanoma cells are influenced by circulating systemic factors such as insulin, which we recently demonstrated acts to modulate melanocyte proliferation and invasion via the sheddase BACE2 (Zhang et al. Developmental Cell 2018).
Ongoing work: We have significant efforts aimed at understanding how keratinocytes and adipocytes influence melanoma progression. A major focus is how cross-talk between these cells affects the epigenetic/transcriptional state of the cell. This may occur via metabolic rewiring from fatty acids or through other secreted factors.
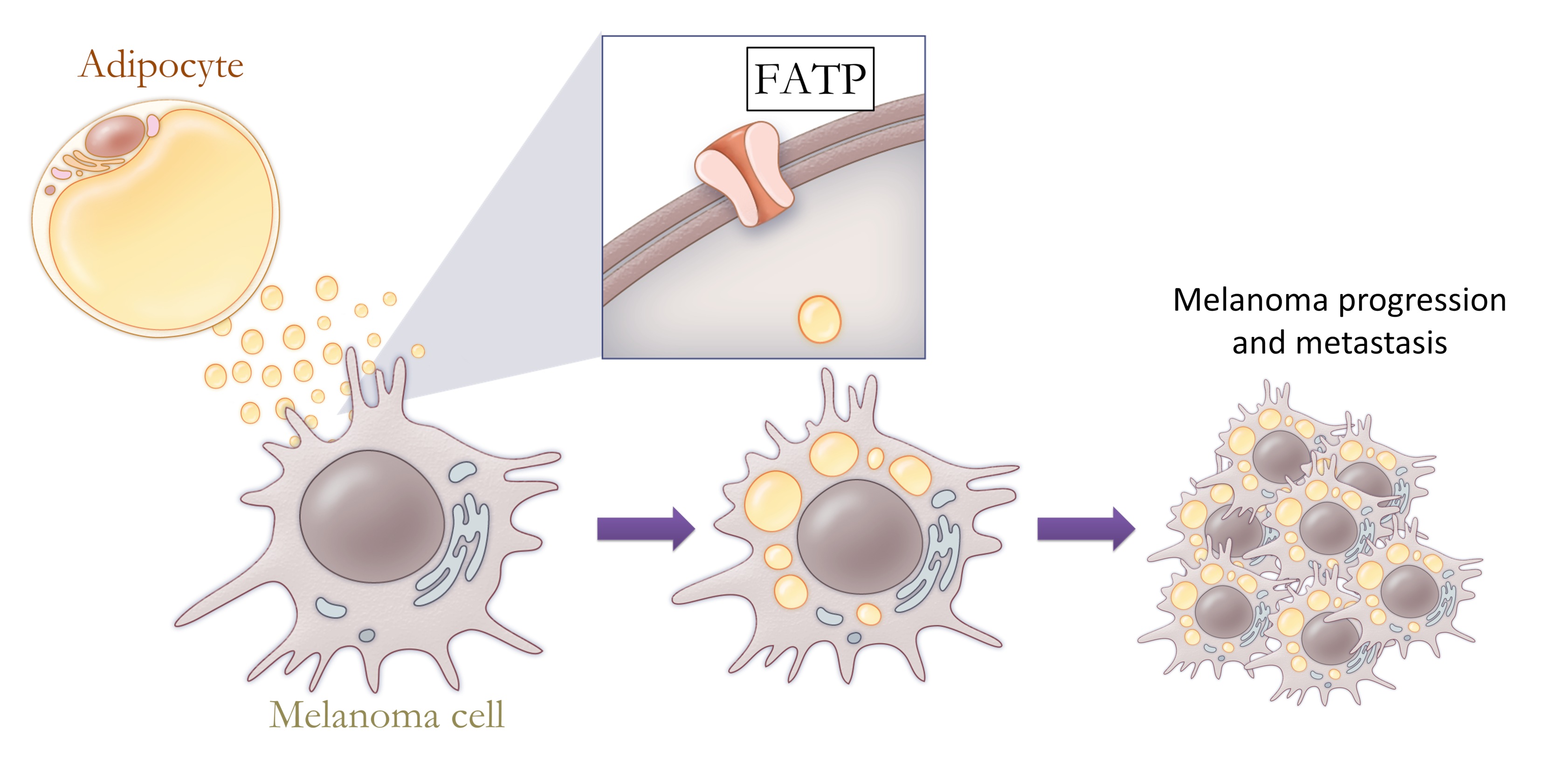
Adipocytes in the microenvironment can provide fatty acids to melanoma cells, fueling tumor progression and metastasis
Identifying the network of tumor/microenvironment interactions in metastatic progression
Within both the tumor and microenvironment, there is tremendous heterogeneity. While some of this heterogeneity is genetic, less well explored is the range of transcriptional heterogeneity within tumor and microenvironmental cells. We have used single cell RNA-seq to identify all of the cell types present in our zebrafish melanomas (Baron, et al), revealing a surprising diversity of cell types present in our tumors. We are using small molecule and CRISPR approaches to dissect these interactions, which will provide a wealth of new mechanisms by which these cell-cell interactions can coordinately promote metastatic progression.
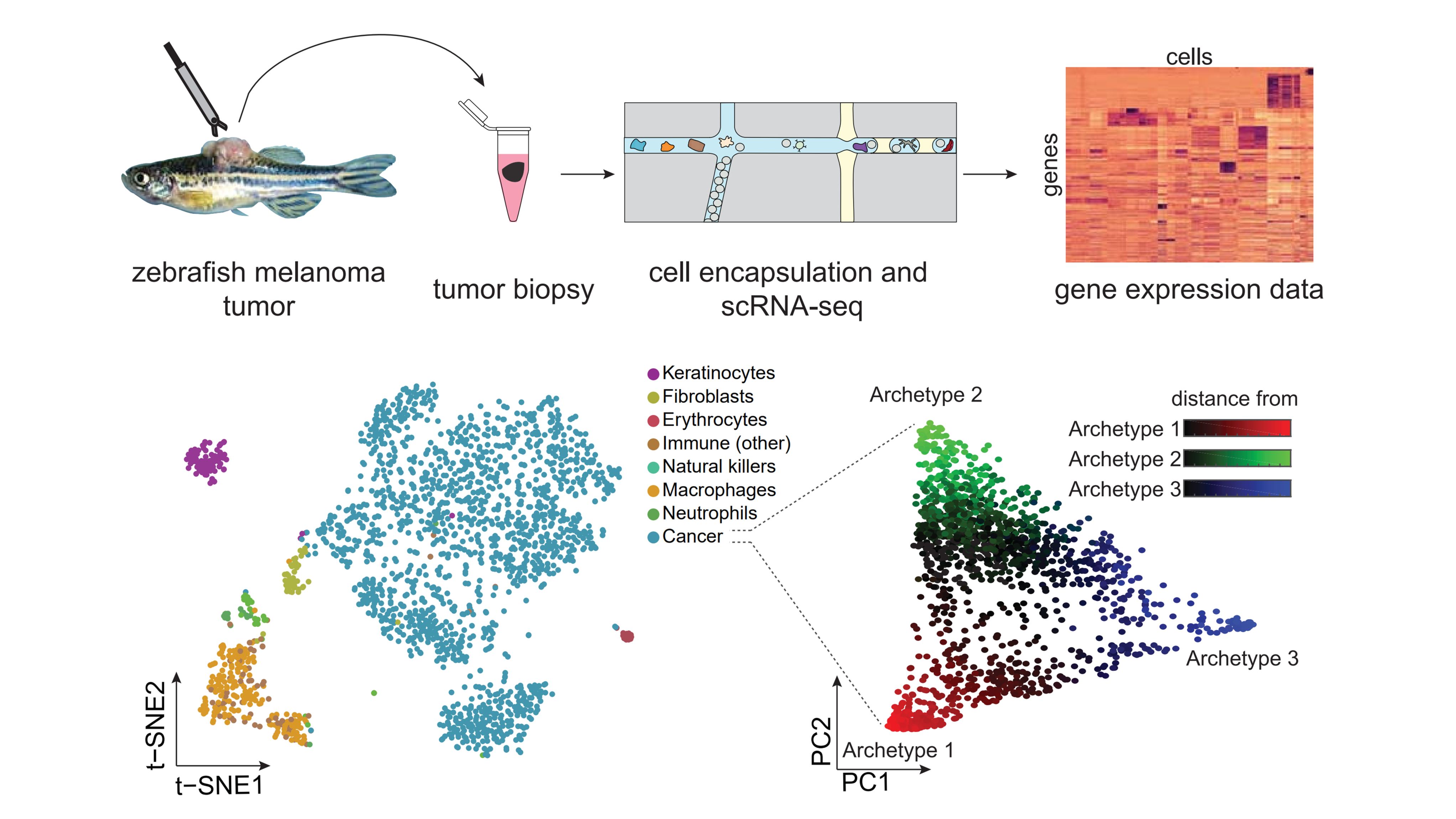
Zebrafish melanomas were subjected to repeated sampling and single-cell sequencing, uncovering the range of both tumor and microenvironmental cell types present in the tumor.