Luis Parada suspects there are several types of glioblastoma that require different types of treatment.
Finding a treatment for brain tumors like glioblastoma (GBM) has been a heartbreaking challenge. The five-year survival rate is less than 10% for patients 45 and older. Because these tumors are relatively rare and located in such a sensitive and well-sheltered part of the body, researchers have lacked a reliable source of tumor tissue to understand their underlying biology.
No one knows this better than Sloan Kettering Institute (SKI) developmental biologist Luis Parada, who has spent much of his scientific career trying to study the elusive biology of these tumors. Dr. Parada, who holds the Albert C. Foster Chair, came to Memorial Sloan Kettering Cancer Center in 2015 with the explicit goal of developing a better way.
“My excitement and vision for coming here was to build a really comprehensive patient-derived xenograft program,” Dr. Parada says.
A patient-derived xenograft (PDX) is a laboratory model in which cells from a human tumor are transplanted into a research animal, such as a mouse. The human cells grow into a new tumor in the animal and can then be studied on the microscopic level to better understand their properties and also used to test potential drugs.
As Director of MSK’s Brain Tumor Center (BTC), Dr. Parada collaborates with clinicians and scientists throughout the institution, breaking down silos to advance their understanding. As a result, the Center now has created more than 100 verified PDX models, ready to be studied by researchers at MSK.
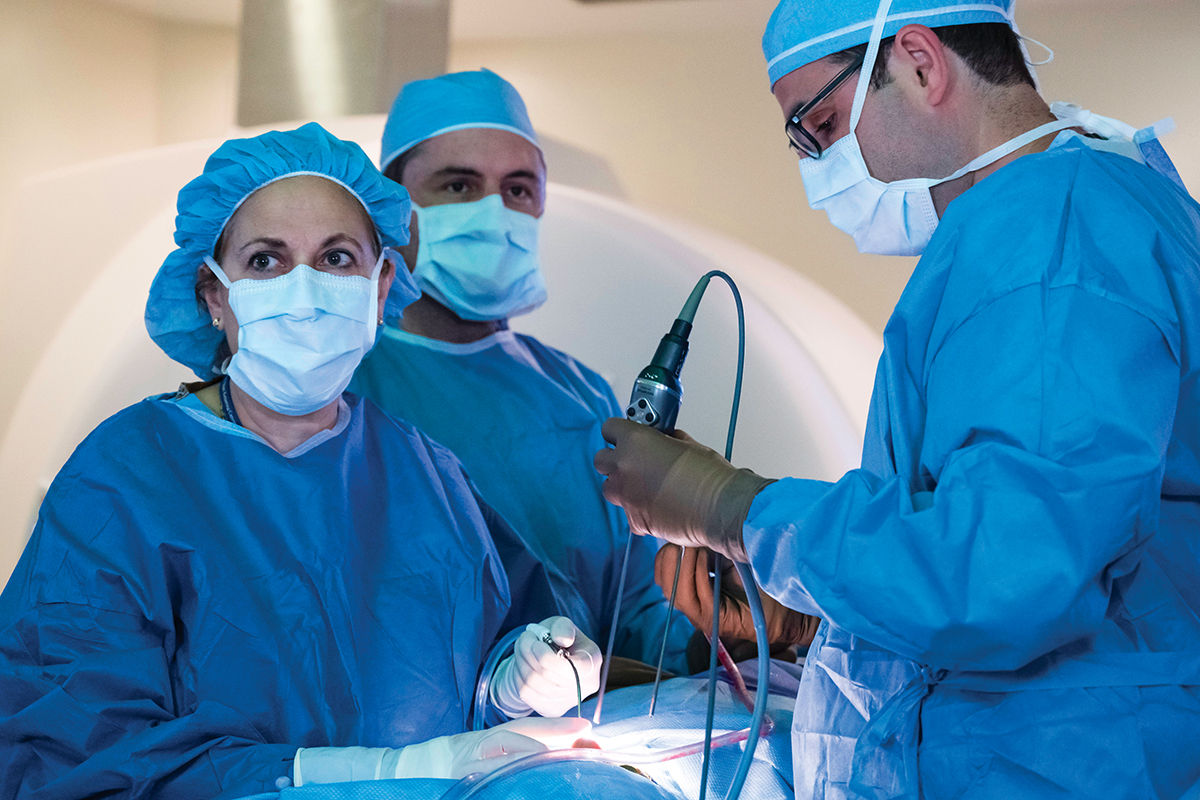
Neurosurgeon Viviane Tabar sends patient tumor samples from the operating room directly to the lab.
Dr. Parada’s protocol for creating these models is among the most stringent compared with programs at other institutions. The human tumor cells for his models come directly from patients who are having surgery at MSK. Neurosurgeons and affiliated faculty members of the BTC, Viviane Tabar, who holds the Theresa Feng Chair in Neurosurgery, and Cameron Brennan perform most of these surgeries at Memorial Hospital.
Once the tumor tissue is removed from the patient, it is quickly whisked away by Dr. Parada’s team to be surgically implanted directly into the brain of a mouse. The tumor cells never touch a culture dish or other chemicals that might alter their activity. Moreover, they are grown in the same organ from which they were derived — the brain. Then, Dr. Parada’s team monitors them through MRI scans to make sure that they have taken root in the mouse brains.
MSK is revolutionizing the way cancer is diagnosed and treated. Learn more about how your generosity makes this possible.
Once three successive generations of mice have been grown with the transplanted tumors, the researchers sequence the tumor’s DNA to make sure that the PDX tumor is genetically the same as the original tumor from the patient. Only after all these steps are complete is the PDX model considered validated and ready for research.
“To my knowledge, no other program is as comprehensive, as meticulous and rigorous, as the one we have at Memorial Sloan Kettering,” Dr. Parada says.
Neuro-oncologist Ingo Mellinghoff specializes in the treatment of primary brain tumors.
He and the other members of the BTC, including physician-scientist Ingo Mellinghoff, who is Chair of the Department of Neurology and holds the Evnin Family Chair in Neuro-Oncology, hope their carefully curated PDX models will finally enable researchers to make real progress in treating patients with these deadly tumors.
Mice as Surrogates for Patients
Researchers are using the PDX models to establish various subtypes of glioblastoma based on their genetic makeup. Then, these tumors in mice are treated with drugs in what they call a “preclinical trial” — similar to what you would do with patients, but with mice instead.
“That’s unprecedented,” Dr. Parada says. “These PDX models are as close as you can get to actually experimenting on human brain tumors.”
Dr. Parada suspects that there isn’t one glioblastoma, but rather five or six subtypes, based on preliminary evidence. His goal is to identify unique vulnerabilities of each subtype and develop specific treatments for each of them. One day, the team at MSK’s Brain Tumor Center hopes their work will improve patients’ odds of surviving this deadly disease.
Of course, none of this hope would be possible without the philanthropic investments that made this unique PDX program possible — and keep it running. In particular, Dr. Parada is grateful to the Mortimer B. Zuckerman Family Foundation, Richard A. and Susan P. Friedman, and the Richard H. Schneider Family Fund for making transformational gifts to support brain cancer research initiatives at MSK.