Researchers Jorge Reis-Filho and Britta Weigelt collaborate on cancer genomics research.
Precision oncology is based on the idea that the molecular changes driving cancer can be targeted with drugs to stop a tumor from growing. But what happens if not all of the cells in a single tumor are driven by the same set of mutations? This phenomenon is called tumor heterogeneity, and it’s extremely common in cancer.
Researchers want to get around this problem with an approach called single-cell analysis. This allows them to analyze tumors with greater detail than ever before. Being able to find the genetic mutations that direct individual cells within a tumor may lead to more-effective targeted therapies. Plus, single-cell analysis can help doctors learn when some of the cells in a tumor have begun to develop new mutations that allow them to resist targeted therapies.
“Genetic heterogeneity within tumors is one of the main challenges that we face in precision oncology,” says Memorial Sloan Kettering experimental pathologist Jorge Reis-Filho. His laboratory focuses on the genes that drive the formation and growth of breast cancer. “We use single-cell genomics to understand heterogeneity within tumors and how it contributes to disease progression,” he says.
Looking for Changes in Many Cells
Single-cell analysis is being developed hand in hand with another technology known as liquid biopsy. This technique searches the blood for tumor cells or free-floating DNA that has escaped from a tumor. In many cases, liquid biopsy lets doctors and researchers see a broad range of genetic changes across an entire tumor. This can provide them with much more information than they might get from a tissue biopsy taken from a single area of a tumor.
In spelling out the DNA sequences for many, many individual cells, these studies create a huge amount of data. For that reason, computational biology and bioinformatics are an important part of this field of research. Dana Pe’er, Chair of the Sloan Kettering Institute’s Computational and Systems Biology Program, is leading this effort at MSK. She and her team are creating mathematical algorithms that allow computers to scan the large amounts of data that come from single-cell analysis, providing a filter to identify the most important findings.
These approaches are expensive and are not used as a regular part of cancer care. But researchers are looking at ways to reduce the costs. The goal is that these tools will eventually become widely available and the standard way to diagnose cancer and monitor how well treatment is working.
An Important Laboratory Tool for DCIS and Beyond
Currently, Dr. Reis-Filho and his team are using single-cell analysis to study tumor samples from surgical biopsies. In February 2017, they reported that they had developed the first method for doing single-cell sequencing from older tumor samples — even those that have been preserved and stored for years.
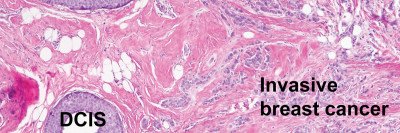
At the San Antonio Breast Cancer Symposium in December 2017, Dr. Reis-Filho discussed research in which he used this technique to study preserved tumor samples from a type of breast tumor called ductal carcinoma in situ (DCIS). DCIS is a very early-stage, noninvasive cancer. Women who develop it have about a 25% chance that the cancer will come back after surgery, and then it can become invasive.
The long-term goal of this work is to find genetic changes in individual cells that predict which people are more likely to have the tumor return and to define if the genetic heterogeneity itself could predict the behavior of DCIS. That way some women with DCIS could receive more-aggressive treatment right away, while others who are not at a high risk could avoid it.
“One interesting finding from our study was that intratumor heterogeneity can occur very early in the formation of a tumor, even in the DCIS stage,” says Dr. Reis-Filho, who is a member of MSK’s Human Oncology and Pathogenesis Program. “This is contrary to what was previously believed — that heterogeneity was an event that developed later in the growth of tumors.” This discovery uncovered new clues about the genetic underpinnings of this type of cancer.
The problem of intratumor heterogeneity is not limited to breast cancer. “We know more about it in types of cancer that have been more extensively studied, like breast cancer and lung cancer,” Dr. Reis-Filho adds. “But we suspect that it applies to most types of solid tumors.”
Investigators continue to advance their ability to study tumors at the level of a single cell. Through this research, they expect to find even more information about what drives tumor growth. This, in turn, will help them improve ways to diagnose cancer. Single-cell analysis may also enable the development of more-effective targeted therapies or combinations of targeted therapies to treat the most-aggressive cells within a tumor.