The Metastasis Highway. Cancer cells can spread to other locations in the body by way of blood vessels (red and purple) as well as lymphatic vessels (yellow). Image credit: Carol and Mike Werner/Science Source
Early Detection of Ovarian Cancer
Ovarian cancer is difficult to treat at an advanced stage, when it is typically diagnosed. There is an urgent need for ways to detect the disease earlier. Daniel Heller, a chemist in the Molecular Pharmacology Program at the Sloan Kettering Institute, and his colleagues have developed a small implantable sensor that shows promise for detecting an early warning sign for ovarian cancer.
The sensor is made of carbon nanotubes, tiny tubes so small that millions of them could fit on the head of a pin. The nanotubes give off an infrared light that passes harmlessly through the body. When the nanotubes encounter a protein called HE4, which is a marker for ovarian cancer, the color of the light changes. By analyzing this light, researchers can measure the HE4 level.
“It gives a reading in just a few seconds, like using a supermarket checkout scanner,” Dr. Heller says.
At the annual meeting of the American Association for Cancer Research (AACR), Dr. Heller’s team described animal tests showing that the sensor reliably detected HE4 in mouse models for ovarian cancer. For people, the researchers envision using the nanotubes in an IUD-type device.
In the short term, this technology might be used in women known to be at a higher risk for ovarian cancer, such as those who carry BRCA mutations, or to check if cancer has come back in women who have already been treated. It could also be used in an ongoing way to monitor treatment.
“Further down the line, we might be able to make a wristwatch-type device that detects many different markers all at the same time — a kind of Fitbit or Apple Watch for disease,” he explains.
This research was funded by Tina’s Wish, the National Institutes of Health, and the Ovarian Cancer Research Fund.
Evaluating a New Immunotherapy Drug, Alone and in Combination
Despite the success of immunotherapy in treating some types of cancer, not everyone responds to this approach. Researchers at Memorial Sloan Kettering and elsewhere are searching for ways to improve these treatments. One promising avenue is combining existing drugs with new ones that attack a different weakness in cancer’s armor or boost the immune system in another way.
At AACR, Roberta Zappasodi, a Parker Institute for Cancer Immunotherapy Bridge Scholar and a Ludwig researcher at MSK, presented results from studies that she, Taha Merghoub, and other members of Jedd Wolchok’s team at MSK conducted on an immune protein called GITR. Previous laboratory studies had suggested that this protein could be a good target for immunotherapy. Antibodies stimulating it led to effective tumor killing in mice. On the basis of this earlier work, Dr. Wolchok and other MSK investigators initiated a phase I clinical trial in people with advanced cancer. The purpose of the study, which was sponsored by the Ludwig Center at MSK and the Cancer Research Institute, was to determine a safe dose of the GITR-targeting drug.

MSK research fellow Roberta Zappasodi.
On April 1, Dr. Zappasodi presented the clinical results from 43 patients as well as companion studies performed on blood samples and in mice. At the dose levels tested, patients did not experience a noticeable improvement in their cancers from the GITR-targeting drug. However, the drug did reduce the amount of regulatory T cells (Tregs) in and around tumors. (These suppressive immune cells can sometimes prevent immunotherapy drugs from working.)
“These results indicated that removing immunosuppressive T cells from the tumor microenvironment is not enough to treat advanced tumors,” Dr. Zappasodi says. “That pushed us to study in animal models how to exploit the immune effects of anti-GITR therapy in rational combinations with other immunotherapies to maximize anti-tumor activity.”
In experiments done in mice, she and her colleagues observed that the combination of anti-GITR plus anti-PD-1 drugs was more effective than either drug given alone. Together, the drugs blocked both Treg suppression and T cell exhaustion. The researchers argue that these findings make a good case for combining anti-GITR drugs with already approved anti-PD-1 immunotherapies, such as nivolumab (Opdivo®) and pembrolizumab (Keytruda®). A clinical trial testing these combinations is already open and accruing patients.
“It would be easy to shy away from GITR as a drug target on the basis of these initial results, but that would be a shame,” says Dr. Merghoub, who is Co-Director of the Ludwig Collaborative Laboratory at MSK and a corresponding author on the abstract. “We should take advantage of these studies to understand the biological activity of these drugs instead. Our studies looking at the biological mechanisms provide a clear rationale for testing GITR-targeting drugs in combination with other immunotherapies.”
The investigators published these results in Nature Medicine.
This research was funded by the National Institutes of Health, Swim Across America, the Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, the Cancer Research Institute, and the Breast Cancer Research Foundation.
Mapping Metastasis
In 1889, an English surgeon named Stephen Paget observed that where breast cancer spread did not seem random. Certain locations, such as the bones and brain, were much more common. He proposed a botanical analogy to explain this finding: Just as seeds need to fall on fertile ground to grow, tumor cells need to land in an environment hospitable to them as well. The idea became known as the “seed and soil” hypothesis. It is now an accepted explanation for metastasis, which is responsible for 90% of deaths due to cancer.
Although scientists recognize some patterns in how cancer spreads, no one had ever compiled a comprehensive road map of metastasis — until now.
As reported on April 1 at AACR, Francisco Sanchez-Vega and his colleagues in Nikolaus Schultz’s lab at MSK analyzed genomic data from nearly 35,000 patients treated at MSK in combination with anonymous information from the patients’ medical histories. The team assembled the map by scoring how frequently different tumor types spread to specific other locations.
The data confirmed some previously reported anecdotal findings. For example, some organs act as “sinks” — they tend to attract metastatic cells. The liver, lungs, and lymph nodes fall into this category. Other organs, like the esophagus, almost never provide a hospitable home to metastases.
Some results were more surprising. When they parsed the data according to distinct molecular subtypes of cancer, there were often clear differences. For example, HER2-positive breast cancer was more likely to spread to the brain and lungs compared with HER2-negative breast cancer. Certain types of colorectal cancer had much lower rates of metastasis than others.
Unlike a literal road map, this genome-derived atlas doesn’t show the precise route of metastasis — how cells got from one place to another. But it does show what fraction of tumors metastasize to each organ.
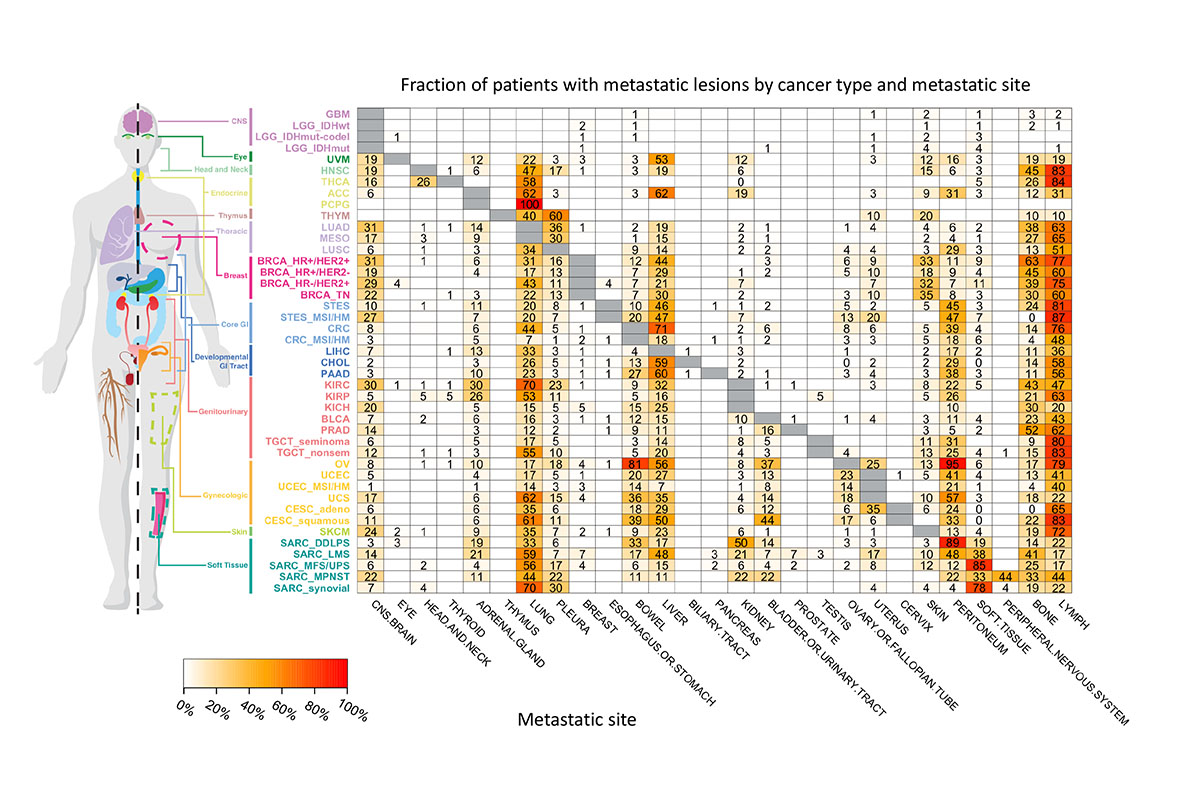
This image shows the fraction of total patients with a particular type of cancer that spread to a particular organ.
Dr. Sanchez-Vega says their ultimate goal is to create a tool for risk assessment and predicting outcomes.
“Imagine that a patient arrives at the clinic,” he says. “They get MSK-IMPACT™ testing. The hope is that we could use those results to predict the probability of their having metastasis or not. Doctors could then use that information to decide how often to do imaging scans or how aggressively to treat the cancer. Also, in the long term, connecting the clinical and genetic information with the sites to which cancers metastasize may enable doctors to treat some patients with targeted therapies that can prevent metastases to certain highly vulnerable sites, like the brain. This in turn may enable us to improve outcomes for people with cancer, which is really our ultimate goal.”
The investigators received support from the Center for Metastasis Research at MSK and the Brown Performance Fund for Innovation in Cancer Informatics.
CAR T Fratricide
An immunotherapy called chimeric antigen receptor (CAR) T cell therapy has shown exciting promise in treating people with aggressive types of leukemia and lymphoma. Different cancer centers using this approach have achieved complete cancer regression rates that hover around 85% for people with acute lymphoblastic leukemia. Unfortunately, relapses are fairly common. Research from Michel Sadelain’s lab at MSK is shedding light on different ways that cancers can escape from CAR-mediated killing and devising strategies to overcome them.
On April 2 at AACR, Dr. Sadelain will be chairing a session on overcoming resistance in T cell–based therapies, and also presenting results from his own lab. His team has recently found that CAR T cells can sometimes perform an act of antigen stealing called trogocytosis. In this process, the target antigen on cancer cells — CD19 in the case of leukemia and lymphoma — physically moves from a cancer cell to a CAR T cell.
What happens next can be likened to cellular fratricide: Other CAR T cells latch on to their CD19-bearing brothers and kill them. As a result, the level of recognizable CD19 antigen goes down in the body and leads to the cancer escaping detection by the remaining CAR T cells.
MSK research fellow Mohamad Hamieh.
In an accompanying paper published on March 22 in Nature, Dr. Sadelain and first author Mohamad Hamieh describe the experiments they have done in mice to show how this process works. In addition to CD19, they found that CAR-induced trogocytosis occurred with all other tested CAR targets, including CD22, B cell maturation antigen (found on multiple myeloma), and mesothelin. They conclude that trogocytosis is likely a general feature of CAR T cells.
They also describe ways that this fratricidal killing might be avoided to lessen the chance of escape. One approach, they find, is using two types of CAR T cells at the same time, each targeting a different antigen on the cancer cells. The findings will inform the design of upcoming clinical trials for people with acute myeloid leukemia and multiple myeloma, Dr. Sadelain says.
After Dr. Sadelain’s session, MSK’s Christopher Klebanoff will give the talk “T Cell Therapy 2.0: Harder, Better, Faster, Stronger.” He will share how his lab is developing T cell receptor–engineered cells for cancer therapy.
This work was supported by the Lake Road Foundation, the Leukemia and Lymphoma Society, the Pasteur-Weizmann/Servier award, the National Cancer Institute, the Tow Foundation, Cycle for Survival, and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Doctors Beyond Borders
MSK surgeon T. Peter Kingham and surgeon Olusegun Isaac Alatise, of the Obafemi Awolowo University Teaching Hospital, in Ile-Ife, Nigeria, have collaborated since 2010. Dr. Alatise spent several months observing at MSK and learning new surgical techniques. He spoke at AACR about their cooperative efforts and the progress made in increasing surgical capacity and improving cancer care in Nigeria.
The two surgeons founded a ten-hospital cancer consortium in Nigeria with MSK as the global partner. MSK has several initiatives focused on improving diagnosis and care of people with breast cancer and colorectal cancer in that country. At AACR, Dr. Alatise gave an overview of the molecular differences of colorectal cancer in Nigeria compared with cases seen in the United States, and what this means in terms of diagnosis and treatment.
MSK surgeon T. Peter Kingham (right) with surgeon Olusegun Isaac Alatise