Deriving specific brain and spinal cord cell types from human PSCs
Based on conserved developmental principles, we have developed a simple but highly efficient platform to convert human PSCs into neural tissue. We have shown that dual-SMAD inhibition via small molecule inhibitors of BMP and TGFb/Activin/Nodal signaling is sufficient to trigger neural conversion. Using this technology we are particularly interested in studying the specification of midbrain dopamine neurons. Those studies include the identification of molecular pathways and cell type – specific markers to induce or isolate specific subpopulations of dopamine neurons. We are also interested in forebrain development, including the specification of various dorsal and ventral forebrain neurons. Finally, we have a long-standing interest in the identification of defined human PSC-derived neural stem cell (NSC) lineages. We have described rosette-stage NSCs and continue to work on identifying defined NSC stages along the progression from the stage of neural plate formation to gliogenesis.
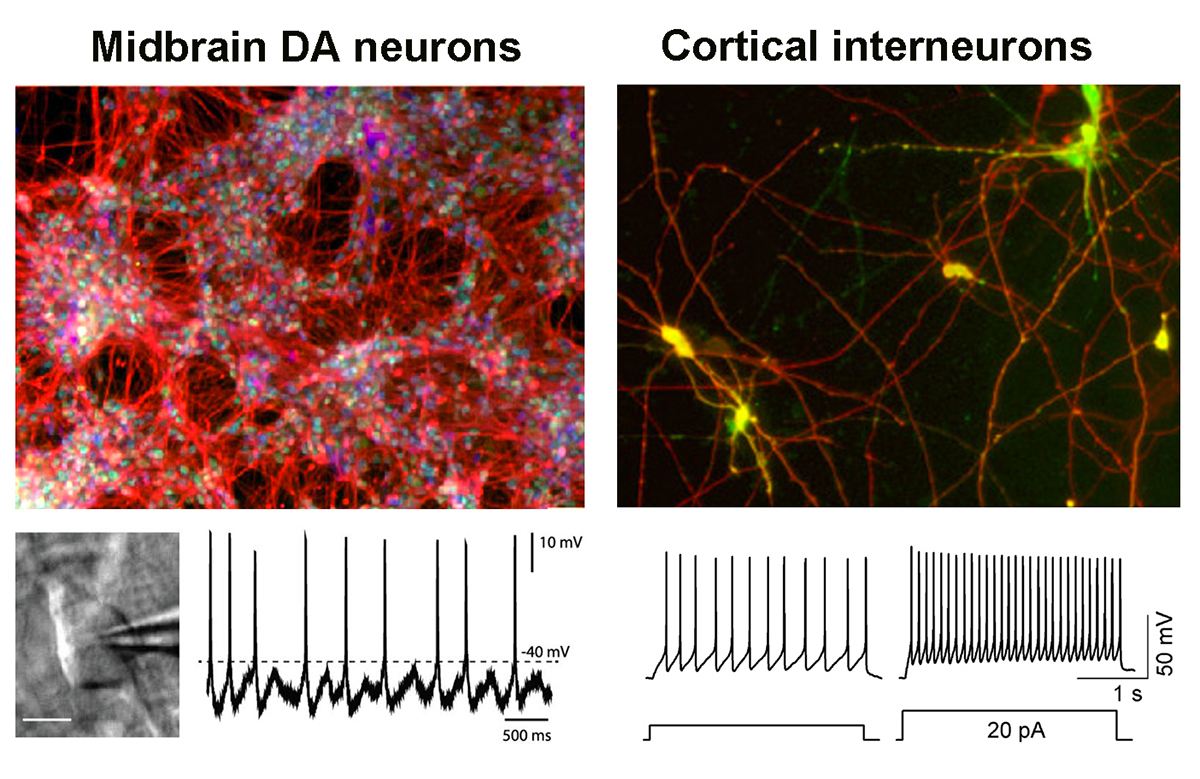
Midbrain dopamine and cortical interneurons — Left panels: Lab-grown dopamine neurons expressing neuronal (red) and midbrain specific (green and blue) markers. Right panels: Lab-grown cortical interneurons from human pluripotent stem cells are tools to study psychiatric disorders. Specific functional, electric firing properties of those neurons are shown by electrophysiology.
Directing neural crest and sensory placode lineages
Multipotent neural crest (NC) precursor cells have the ability to yield a broad range of cell types of both ectodermal and mesenchymal lineage identity. We have described the isolation of multipotent NC precursors from human PSCs using cell surface markers. We have also developed highly directed protocols based on exposure to molecules that activate WNT signaling to obtain NC lineages such as SOX10+ NC precursors, melanocytes, or nociceptive (pain-sensing) sensory neurons, and ongoing studies are trying to extend those efforts toward other NC lineages such as the enteric nervous stem. A developmental structure related to the NC is the cranial placode, giving rise to various cell types in the sensory organs as well as certain cranial nerves such as trigeminal neurons. We have been able to recreate the molecular signaling sufficient to drive sensory placode formation in hPSCs and work on the isolation of various medically important placode derivatives.
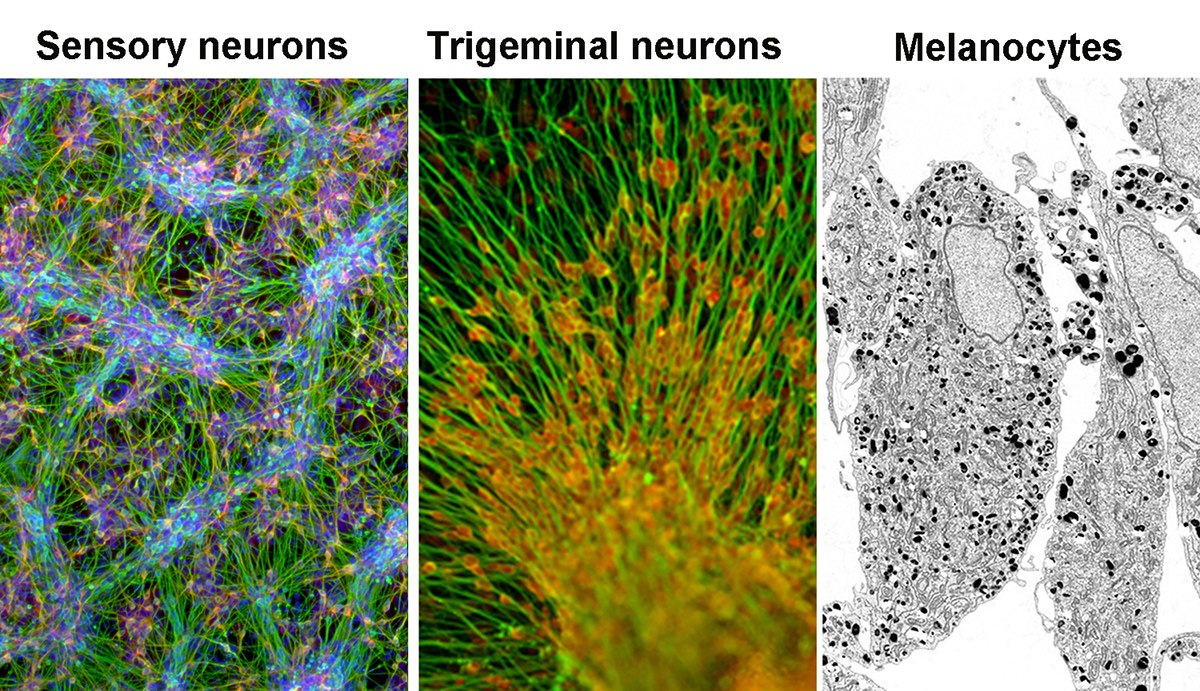
Sensory neurons of the peripheral nervous system and NC-derived melanocytes — Left panel: Cells differentiating into pain-sensing neurons. Middle panel: Immature neurons of the trigeminal nerve derived from hPSCs. Right panel: Electron microscopy image of hPSC-derived melanocyte producing melanin.
Rapid induction of fate, maturation, and age in human PSC-derived lineages
While our ability to direct cell fate from human PSCs has dramatically improved over the last few years, the mechanisms that control the timing of human development and differentiation remain largely a mystery. We have started to address this issue by screening for molecules that can induce neuronal induction in a highly accelerated timeframe, which mimics the timing observed in mouse PSC-derived lineages.
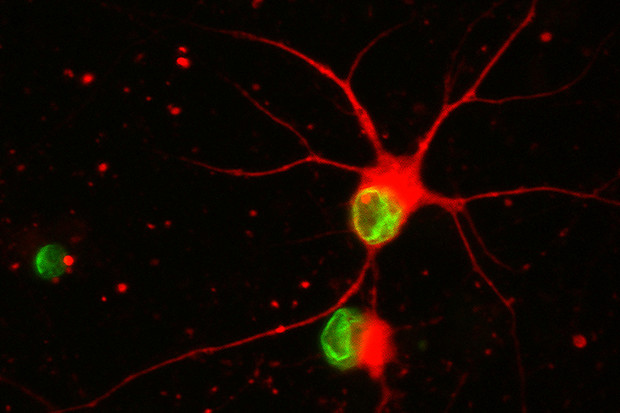
Inducing age-like features in nerve cells — When stem-cell-derived nerve cells exposed to progerin (shown in green) begin to degenerate, their long, branch-like processes progressively shorten, then disappear. The top right cell is still healthy; the cell below it has begun to degenerate. The lefthand cell has lost its processes and is dying.